Pathological consequences of MICU1 mutations on mitochondrial calcium signalling and bioenergetics
- PMID: 28132899
- PMCID: PMC5424885
- DOI: 10.1016/j.bbamcr.2017.01.015
Pathological consequences of MICU1 mutations on mitochondrial calcium signalling and bioenergetics
Abstract
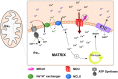
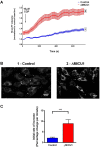
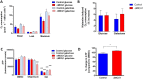
Loss of function mutations of the protein MICU1, a regulator of mitochondrial Ca2+ uptake, cause a neuronal and muscular disorder characterised by impaired cognition, muscle weakness and an extrapyramidal motor disorder. We have shown previously that MICU1 mutations cause increased resting mitochondrial Ca2+ concentration ([Ca2+]m). We now explore the functional consequences of MICU1 mutations in patient derived fibroblasts in order to clarify the underlying pathophysiology of this disorder. We propose that deregulation of mitochondrial Ca2+ uptake through loss of MICU1 raises resting [Ca2+]m, initiating a futile Ca2+ cycle, whereby continuous mitochondrial Ca2+ influx is balanced by Ca2+ efflux through the sodium calcium exchanger (NLCXm). Thus, inhibition of NCLXm by CGP-37157 caused rapid mitochondrial Ca2+ accumulation in patient but not control cells. We suggest that increased NCLX activity will increase sodium/proton exchange, potentially undermining oxidative phosphorylation, although this is balanced by dephosphorylation and activation of pyruvate dehydrogenase (PDH) in response to the increased [Ca2+]m. Consistent with this model, while ATP content in patient derived or control fibroblasts was not different, ATP increased significantly in response to CGP-37157 in the patient but not the control cells. In addition, EMRE expression levels were altered in MICU1 patient cells compared to the controls. The MICU1 mutations were associated with mitochondrial fragmentation which we show is related to altered DRP1 phosphorylation. Thus, MICU1 serves as a signal-noise discriminator in mitochondrial calcium signalling, limiting the energetic costs of mitochondrial Ca2+ signalling which may undermine oxidative phosphorylation, especially in tissues with highly dynamic energetic demands. This article is part of a Special Issue entitled: ECS Meeting edited by Claus Heizmann, Joachim Krebs and Jacques Haiech.
Keywords: Calcium; MICU1; Mitochondria; PDH.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures



References
-
- Lehninger A.L., Reynafarje B., Vercesi A., Tew W.P. Transport and accumulation of calcium in mitochondria. Ann. N. Y. Acad. Sci. 1978;307:160–176. - PubMed
-
- Crompton M.S., Sigel E., Salzmann M., Carafoli E. A kinetic study of the energy-linked influx of Ca2+ into heart mitochondria. Eur. J. Biochem. 1976;69
-
- King M.P., Attardi G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science. 1989;246:500–503. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

