Cellular retinoid binding-proteins, CRBP, CRABP, FABP5: Effects on retinoid metabolism, function and related diseases
- PMID: 28132904
- PMCID: PMC5408321
- DOI: 10.1016/j.pharmthera.2017.01.004
Cellular retinoid binding-proteins, CRBP, CRABP, FABP5: Effects on retinoid metabolism, function and related diseases
Abstract
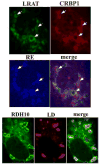
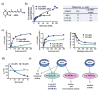
Cellular binding-proteins (BP), including CRBP1, CRBP2, CRABP1, CRABP2, and FABP5, shepherd the poorly aqueous soluble retinoids during uptake, metabolism and function. Holo-BP promote efficient use of retinol, a scarce but essential nutrient throughout evolution, by sheltering it and its major metabolite all-trans-retinoic acid from adventitious interactions with the cellular milieu, and by imposing specificity of delivery to enzymes, nuclear receptors and other partners. Apo-BP reflect cellular retinoid status and modify activities of retinoid metabolon enzymes, or exert non-canonical actions. High ligand binding affinities and the nature of ligand sequestration necessitate external factors to prompt retinoid release from holo-BP. One or more of cross-linking, kinetics, and colocalization have identified these factors as RDH, RALDH, CYP26, LRAT, RAR and PPARβ/δ. Michaelis-Menten and other kinetic approaches verify that BP channel retinoids to select enzymes and receptors by protein-protein interactions. Function of the BP and enzymes that constitute the retinoid metabolon depends in part on retinoid exchanges unique to specific pairings. The complexity of these exchanges configure retinol metabolism to meet the diverse functions of all-trans-retinoic acid and its ability to foster contrary outcomes in different cell types, such as inducing apoptosis, differentiation or proliferation. Altered BP expression affects retinoid function, for example, by impairing pancreas development resulting in abnormal glucose and energy metabolism, promoting predisposition to breast cancer, and fostering more severe outcomes in prostate cancer, ovarian adenocarcinoma, and glioblastoma. Yet, the extent of BP interactions with retinoid metabolon enzymes and their impact on retinoid physiology remains incompletely understood.
Keywords: Cellular retinoic acid binding-protein; Cellular retinol binding-protein; Retinal dehydrogenase; Retinoic acid; Retinoids; Retinol; Retinol dehydrogenase.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
The author declares that there are no conflicts of interest.
Figures

Similar articles
-
Retinoid metabolism and functions mediated by retinoid binding-proteins.Methods Enzymol. 2020;637:55-75. doi: 10.1016/bs.mie.2020.02.004. Epub 2020 Apr 1. Methods Enzymol. 2020. PMID: 32359659 Free PMC article.
-
Functions of Intracellular Retinoid Binding-Proteins.Subcell Biochem. 2016;81:21-76. doi: 10.1007/978-94-024-0945-1_2. Subcell Biochem. 2016. PMID: 27830500 Free PMC article. Review.
-
Molecular and metabolic retinoid pathways in the human ocular surface.Mol Vis. 2007 Sep 11;13:1641-50. Mol Vis. 2007. PMID: 17893666
-
Cellular retinoid-binding proteins transfer retinoids to human cytochrome P450 27C1 for desaturation.J Biol Chem. 2021 Oct;297(4):101142. doi: 10.1016/j.jbc.2021.101142. Epub 2021 Sep 1. J Biol Chem. 2021. PMID: 34480899 Free PMC article.
-
Enzymes and binding proteins affecting retinoic acid concentrations.J Steroid Biochem Mol Biol. 1995 Jun;53(1-6):497-502. doi: 10.1016/0960-0760(95)00096-i. J Steroid Biochem Mol Biol. 1995. PMID: 7626500 Review.
Cited by
-
Current Trends in ATRA Delivery for Cancer Therapy.Pharmaceutics. 2020 Jul 28;12(8):707. doi: 10.3390/pharmaceutics12080707. Pharmaceutics. 2020. PMID: 32731612 Free PMC article. Review.
-
Transcriptomic Profiles of Pectoralis major Muscles Affected by Spaghetti Meat and Woody Breast in Broiler Chickens.Animals (Basel). 2024 Jan 5;14(2):176. doi: 10.3390/ani14020176. Animals (Basel). 2024. PMID: 38254345 Free PMC article.
-
All trans retinoic acid as a host-directed immunotherapy for tuberculosis.Curr Res Immunol. 2022 Mar 30;3:54-72. doi: 10.1016/j.crimmu.2022.03.003. eCollection 2022. Curr Res Immunol. 2022. PMID: 35496824 Free PMC article. Review.
-
Single-cell transcriptomics reveals gene expression dynamics of human fetal kidney development.PLoS Biol. 2019 Feb 21;17(2):e3000152. doi: 10.1371/journal.pbio.3000152. eCollection 2019 Feb. PLoS Biol. 2019. PMID: 30789893 Free PMC article.
-
Attenuation of Hypertrophy in Human MSCs via Treatment with a Retinoic Acid Receptor Inverse Agonist.Int J Mol Sci. 2020 Feb 20;21(4):1444. doi: 10.3390/ijms21041444. Int J Mol Sci. 2020. PMID: 32093330 Free PMC article.
References
-
- Allegretto EA, McClurg MR, Lazarchik SB, Clemm DL, Kerner SA, Elgort MG, … Heyman RA. Transactivation properties of retinoic acid and retinoid X receptors in mammalian cells and yeast. Correlation with hormone binding and effects of metabolism. The Journal of Biological Chemistry. 1993;268(35):26625–26633. - PubMed
-
- Barbus S, Tews B, Karra D, Hahn M, Radlwimmer B, Delhomme N, … Lichter P. Differential retinoic acid signaling in tumors of long- and short-term glioblastoma survivors. Journal of the National Cancer Institute. 2011;103(7):598–606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

