Cellular retinoid binding-proteins, CRBP, CRABP, FABP5: Effects on retinoid metabolism, function and related diseases
- PMID: 28132904
- PMCID: PMC5408321
- DOI: 10.1016/j.pharmthera.2017.01.004
Cellular retinoid binding-proteins, CRBP, CRABP, FABP5: Effects on retinoid metabolism, function and related diseases
Abstract
Cellular binding-proteins (BP), including CRBP1, CRBP2, CRABP1, CRABP2, and FABP5, shepherd the poorly aqueous soluble retinoids during uptake, metabolism and function. Holo-BP promote efficient use of retinol, a scarce but essential nutrient throughout evolution, by sheltering it and its major metabolite all-trans-retinoic acid from adventitious interactions with the cellular milieu, and by imposing specificity of delivery to enzymes, nuclear receptors and other partners. Apo-BP reflect cellular retinoid status and modify activities of retinoid metabolon enzymes, or exert non-canonical actions. High ligand binding affinities and the nature of ligand sequestration necessitate external factors to prompt retinoid release from holo-BP. One or more of cross-linking, kinetics, and colocalization have identified these factors as RDH, RALDH, CYP26, LRAT, RAR and PPARβ/δ. Michaelis-Menten and other kinetic approaches verify that BP channel retinoids to select enzymes and receptors by protein-protein interactions. Function of the BP and enzymes that constitute the retinoid metabolon depends in part on retinoid exchanges unique to specific pairings. The complexity of these exchanges configure retinol metabolism to meet the diverse functions of all-trans-retinoic acid and its ability to foster contrary outcomes in different cell types, such as inducing apoptosis, differentiation or proliferation. Altered BP expression affects retinoid function, for example, by impairing pancreas development resulting in abnormal glucose and energy metabolism, promoting predisposition to breast cancer, and fostering more severe outcomes in prostate cancer, ovarian adenocarcinoma, and glioblastoma. Yet, the extent of BP interactions with retinoid metabolon enzymes and their impact on retinoid physiology remains incompletely understood.
Keywords: Cellular retinoic acid binding-protein; Cellular retinol binding-protein; Retinal dehydrogenase; Retinoic acid; Retinoids; Retinol; Retinol dehydrogenase.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
The author declares that there are no conflicts of interest.
Figures
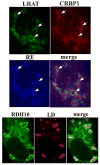
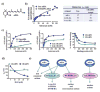

References
-
- Allegretto EA, McClurg MR, Lazarchik SB, Clemm DL, Kerner SA, Elgort MG, … Heyman RA. Transactivation properties of retinoic acid and retinoid X receptors in mammalian cells and yeast. Correlation with hormone binding and effects of metabolism. The Journal of Biological Chemistry. 1993;268(35):26625–26633. - PubMed
-
- Barbus S, Tews B, Karra D, Hahn M, Radlwimmer B, Delhomme N, … Lichter P. Differential retinoic acid signaling in tumors of long- and short-term glioblastoma survivors. Journal of the National Cancer Institute. 2011;103(7):598–606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

