Therapeutic potential of the heme oxygenase-1 inducer hemin against Ebola virus infection
- PMID: 28133423
- PMCID: PMC5267496
Therapeutic potential of the heme oxygenase-1 inducer hemin against Ebola virus infection
Abstract
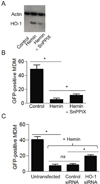
Promising drugs to treat Ebola virus (EBOV) infection are currently being developed, but so far none has shown efficacy in clinical trials. Drugs that can stimulate host innate defense responses may retard the progression of EBOV disease. We report here the dramatic effect of hemin, the natural inducer of the heme catabolic enzyme heme oxygenase-1 (HO-1), in the reduction of EBOV replication. Treatment of primary monocyte-derived macrophages (MDM), Vero E6 cells, HeLa cells, and human foreskin fibroblasts (HFF1) with hemin reduced EBOV infection by >90%, and showed minimal toxicity to infected cells. Inhibition of HO-1 enzymatic activity and silencing HO-1 expression prevented the hemin-mediated suppression of EBOV infection, suggesting an important role for induction of this intracellular mediator in restricting EBOV replication. The inverse correlation between hemin-induced HO-1 and EBOV replication provides a potentially useful therapeutic modality based on the stimulation of an innate cellular response against Ebola infection.
Keywords: Ebola; heme oxygenase-1; hemin; monocytes/macrophages.
Conflict of interest statement
STATEMENT The authors declare no conflict of interest.
Figures

References
-
- Sanchez A, Geisbert TW, Feldmann H. Filoviridae: Marburg and Ebola Viruses. Philadelphia: Fields Virology Lippincott-Williams & Wilkins; 2007.
-
- Ascenzi P, Bocedi A, Heptonstall J, Capobianchi MR, Caro AD, Mastrangelo E, Bolognesi M, Ippolito G. Mol. Aspects Med. 2008;29:151–185. - PubMed
-
- Zhang Y, Feng S, Cowling BJ. Lancet. 2016;387:509. - PubMed
-
- Moll R, Reece S, Cosford P, Kessel A. Br. Med. Bull. 2016;117:15–23. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
