Differences in the Cellular Response to Acute Spinal Cord Injury between Developing and Mature Rats Highlights the Potential Significance of the Inflammatory Response
- PMID: 28133446
- PMCID: PMC5233684
- DOI: 10.3389/fncel.2016.00310
Differences in the Cellular Response to Acute Spinal Cord Injury between Developing and Mature Rats Highlights the Potential Significance of the Inflammatory Response
Abstract
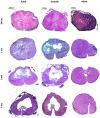
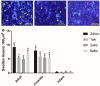
There exists a trend for a better functional recovery from spinal cord injury (SCI) in younger patients compared to adults, which is also reported for animal studies; however, the reasons for this are yet to be elucidated. The post injury tissue microenvironment is a complex milieu of cells and signals that interact on multiple levels. Inflammation has been shown to play a significant role in this post injury microenvironment. Endogenous neural progenitor cells (NPC), in the ependymal layer of the central canal, have also been shown to respond and migrate to the lesion site. This study used a mild contusion injury model to compare adult (9 week), juvenile (5 week) and infant (P7) Sprague-Dawley rats at 24 h, 1, 2, and 6 weeks post-injury (n = 108). The innate cells of the inflammatory response were examined using counts of ED1/IBA1 labeled cells. This found a decreased inflammatory response in the infants, compared to the adult and juvenile animals, demonstrated by a decreased neutrophil infiltration and macrophage and microglial activation at all 4 time points. Two other prominent cellular contributors to the post-injury microenvironment, the reactive astrocytes, which eventually form the glial scar, and the NPC were quantitated using GFAP and Nestin immunohistochemistry. After SCI in all 3 ages there was an obvious increase in Nestin staining in the ependymal layer, with long basal processes extending into the parenchyma. This was consistent between age groups early post injury then deviated at 2 weeks. The GFAP results also showed stark differences between the mature and infant animals. These results point to significant differences in the inflammatory response between infants and adults that may contribute to the better recovery indicated by other researchers, as well as differences in the overall injury progression and cellular responses. This may have important consequences if we are able to mirror and manipulate this response in patients of all ages; however much greater exploration in this area is required.
Keywords: age-related; endogenous neural progenitor cells; inflammation; innate immune cells; microglia; neonates; spinal cord injury.
Figures








References
-
- Baldwin S. A., Broderick R., Blades D. A., Scheff S. W. (1998). Alterations in temporal/spatial distribution of GFAP-and vimentin-positive astrocytes after spinal cord contusion with the New York University spinal cord injury device. J. Neurotrauma 15, 1015–1026. 10.1089/neu.1998.15.1015 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

