Idiosyncratic Drug-Induced Liver Injury (IDILI): Potential Mechanisms and Predictive Assays
- PMID: 28133614
- PMCID: PMC5241492
- DOI: 10.1155/2017/9176937
Idiosyncratic Drug-Induced Liver Injury (IDILI): Potential Mechanisms and Predictive Assays
Abstract
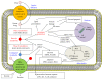
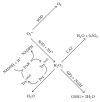
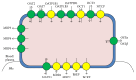
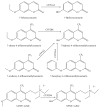
Idiosyncratic drug-induced liver injury (IDILI) is a significant source of drug recall and acute liver failure (ALF) in the United States. While current drug development processes emphasize general toxicity and drug metabolizing enzyme- (DME-) mediated toxicity, it has been challenging to develop comprehensive models for assessing complete idiosyncratic potential. In this review, we describe the enzymes and proteins that contain polymorphisms believed to contribute to IDILI, including ones that affect phase I and phase II metabolism, antioxidant enzymes, drug transporters, inflammation, and human leukocyte antigen (HLA). We then describe the various assays that have been developed to detect individual reactions focusing on each of the mechanisms described in the background. Finally, we examine current trends in developing comprehensive models for examining these mechanisms. There is an urgent need to develop a panel of multiparametric assays for diagnosing individual toxicity potential.
Conflict of interest statement
The authors declare that there are no competing interests regarding the publication of this paper.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

