From Humoral Theory to Performant Risk Stratification in Kidney Transplantation
- PMID: 28133619
- PMCID: PMC5241462
- DOI: 10.1155/2017/5201098
From Humoral Theory to Performant Risk Stratification in Kidney Transplantation
Abstract
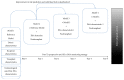
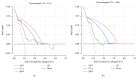
The purpose of the present review is to describe how we improve the model for risk stratification of transplant outcomes in kidney transplantation by incorporating the novel insights of donor-specific anti-HLA antibody (DSA) characteristics. The detection of anti-HLA DSA is widely used for the assessment of pre- and posttransplant risks of rejection and allograft loss; however, not all anti-HLA DSA carry the same risk for transplant outcomes. These antibodies have been shown to cause a wide spectrum of effects on allografts, ranging from the absence of injury to indolent or full-blown acute antibody-mediated rejection. Consequently, the presence of circulating anti-HLA DSA does not provide a sufficient level of accuracy for the risk stratification of allograft outcomes. Enhancing the predictive performance of anti-HLA DSA is currently one of the most pressing unmet needs for facilitating individualized treatment choices that may improve outcomes. Recent advancements in the assessment of anti-HLA DSA properties, including their strength, complement-binding capacity, and IgG subclass composition, significantly improved the risk stratification model to predict allograft injury and failure. Although risk stratification based on anti-HLA DSA properties appears promising, further specific studies that address immunological risk stratification in large and unselected populations are required to define the benefits and cost-effectiveness of such comprehensive assessment prior to clinical implementation.
Conflict of interest statement
The authors declare that they had no financial relationships with any organizations that might have an interest in the submitted work and no other relationships or activities that could appear to have influenced the submitted work.
Figures
Similar articles
-
Evidence for an important role of both complement-binding and noncomplement-binding donor-specific antibodies in renal transplantation.Curr Opin Organ Transplant. 2016 Aug;21(4):433-40. doi: 10.1097/MOT.0000000000000324. Curr Opin Organ Transplant. 2016. PMID: 27348472 Review.
-
Characteristics of Circulating Donor Human Leukocyte Antigen-specific Immunoglobulin G Antibodies Predictive of Acute Antibody-mediated Rejection and Kidney Allograft Failure.Transplantation. 2015 Jun;99(6):1156-64. doi: 10.1097/TP.0000000000000511. Transplantation. 2015. PMID: 25629531 Free PMC article.
-
Association of C1q Binding Status With De Novo HLA Antibody Clinical Features and Allograft Function in Kidney Transplantation Patients During Eight Years of Dynamic Follow-up.Transplant Proc. 2016 Jul-Aug;48(6):1944-54. doi: 10.1016/j.transproceed.2016.05.010. Transplant Proc. 2016. PMID: 27569927
-
Subclass analysis of donor HLA-specific IgG in antibody-incompatible renal transplantation reveals a significant association of IgG4 with rejection and graft failure.Transpl Int. 2015 Dec;28(12):1405-15. doi: 10.1111/tri.12648. Epub 2015 Sep 1. Transpl Int. 2015. PMID: 26264744 Free PMC article.
-
Donor specific HLA antibodies & allograft injury: mechanisms, methods of detection, manifestations and management.Transpl Int. 2018 Oct;31(10):1059-1070. doi: 10.1111/tri.13324. Transpl Int. 2018. PMID: 30062683 Review.
Cited by
-
HLA Desensitization in Solid Organ Transplantation: Anti-CD38 to Across the Immunological Barriers.Front Immunol. 2021 May 20;12:688301. doi: 10.3389/fimmu.2021.688301. eCollection 2021. Front Immunol. 2021. PMID: 34093594 Free PMC article. Review.
-
The Clinical Utility of Post-Transplant Monitoring of Donor-Specific Antibodies in Stable Renal Transplant Recipients: A Consensus Report With Guideline Statements for Clinical Practice.Transpl Int. 2023 Jul 25;36:11321. doi: 10.3389/ti.2023.11321. eCollection 2023. Transpl Int. 2023. PMID: 37560072 Free PMC article.
-
Immune surveillance and humoral immune responses in kidney transplantation - A look back at T follicular helper cells.Front Immunol. 2023 Jul 12;14:1114842. doi: 10.3389/fimmu.2023.1114842. eCollection 2023. Front Immunol. 2023. PMID: 37503334 Free PMC article. Review.
-
Application, technical issues, and interpretation of C1q for graft outcome.Curr Opin Organ Transplant. 2017 Oct;22(5):505-510. doi: 10.1097/MOT.0000000000000454. Curr Opin Organ Transplant. 2017. PMID: 28723698 Free PMC article. Review.
-
Recent advances in kidney transplantation: a viewpoint from the Descartes advisory board.Nephrol Dial Transplant. 2018 Oct 1;33(10):1699-1707. doi: 10.1093/ndt/gfx365. Nephrol Dial Transplant. 2018. PMID: 29342289 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

