Mitochondrial Electron Transport Chain-Derived Superoxide Exits Macrophages: Implications for Mononuclear Cell-Mediated Pathophysiological Processes
- PMID: 28133629
- PMCID: PMC5268359
- DOI: 10.20455/ros.2016.815
Mitochondrial Electron Transport Chain-Derived Superoxide Exits Macrophages: Implications for Mononuclear Cell-Mediated Pathophysiological Processes
Abstract
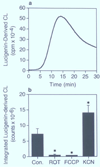
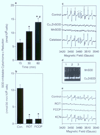
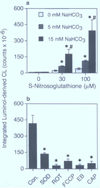
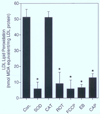
The involvement of mitochondrial electron transport chain (METC)-derived superoxide anion radical in cell protooncogene activation, mitogenic responses, and cancerous growth has recently received much attention. In order for METC-derived superoxide to participate in any of the above processes, its exit from mitochondria would be a critical step. Detection of intracellular superoxide showed that mitochondrial respiration is the major source of cellular superoxide in unstimulated or resting monocytes/macrophages. However, direct evidence for the exit of superoxide from mitochondria is presently lacking. Here we show that METC-derived superoxide does exit from mitochondria in unstimulated monocytes/macrophages. Release of superoxide was first found to occur with substrate-supported mitochondria isolated from these cells. We also observed the presence of extracellular superoxide with the intact unstimulated/resting cells. Extracellular superoxide was markedly diminished (>90%) by the mitochondrial inhibitor, rotenone, or the uncoupler, carbonylcyanide p-(trifluromethy) phenylhydrazone. Furthermore, cells with a deficient METC exhibited significant reduction (>90%) in extracellular superoxide, demonstrating that with intact cells METC-derived superoxide not only exits from mitochondria, but can be released extracellularly. Superoxide anion radical released from mitochondria could react with exogenous nitric oxide, forming peroxynitrite. Mitochondria-derived extracellular superoxide could also oxidize low-density lipoprotein (LDL). These results thus resolve any uncertainty on the ability of superoxide to exit from mitochondria. This study for the first time also identifies mitochondria as the major source of extracellular superoxide in unstimulated resting monocytes/macrophages, which has implications for the involvement of these mononuclear cells in various pathophysiological situations.
Keywords: Chemiluminescence; Electron paramagnetic resonance; Low-density lipoprotein; Macrophages; Mitochondrial electron transport chain; Monocytes; Mononuclear cells; Peroxynitrite; Reactive oxygen species; Superoxide.
Figures



References
-
- Miles PR, Lee P, Trush MA, Van Dyke K. Chemiluminescence associated with phagocytosis of foreign particles in rabbit alveolar macrophages. Life Sci. 1977;20(1):165–170. - PubMed
-
- Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol. 2004;2(10):820–832. - PubMed
-
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–1695. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
