The human longevity gene homolog INDY and interleukin-6 interact in hepatic lipid metabolism
- PMID: 28133767
- PMCID: PMC5519435
- DOI: 10.1002/hep.29089
The human longevity gene homolog INDY and interleukin-6 interact in hepatic lipid metabolism
Abstract
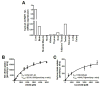
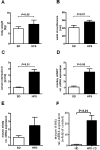
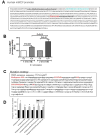
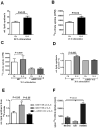
Reduced expression of the Indy ("I am Not Dead, Yet") gene in lower organisms promotes longevity in a manner akin to caloric restriction. Deletion of the mammalian homolog of Indy (mIndy, Slc13a5) encoding for a plasma membrane-associated citrate transporter expressed highly in the liver, protects mice from high-fat diet-induced and aging-induced obesity and hepatic fat accumulation through a mechanism resembling caloric restriction. We studied a possible role of mIndy in human hepatic fat metabolism. In obese, insulin-resistant patients with nonalcoholic fatty liver disease, hepatic mIndy expression was increased and mIndy expression was also independently associated with hepatic steatosis. In nonhuman primates, a 2-year high-fat, high-sucrose diet increased hepatic mIndy expression. Liver microarray analysis showed that high mIndy expression was associated with pathways involved in hepatic lipid metabolism and immunological processes. Interleukin-6 (IL-6) was identified as a regulator of mIndy by binding to its cognate receptor. Studies in human primary hepatocytes confirmed that IL-6 markedly induced mIndy transcription through the IL-6 receptor and activation of the transcription factor signal transducer and activator of transcription 3, and a putative start site of the human mIndy promoter was determined. Activation of the IL-6-signal transducer and activator of transcription 3 pathway stimulated mIndy expression, enhanced cytoplasmic citrate influx, and augmented hepatic lipogenesis in vivo. In contrast, deletion of mIndy completely prevented the stimulating effect of IL-6 on citrate uptake and reduced hepatic lipogenesis. These data show that mIndy is increased in liver of obese humans and nonhuman primates with NALFD. Moreover, our data identify mIndy as a target gene of IL-6 and determine novel functions of IL-6 through mINDY.
Conclusion: Targeting human mINDY may have therapeutic potential in obese patients with nonalcoholic fatty liver disease. German Clinical Trials Register: DRKS00005450. (Hepatology 2017;66:616-630).
© 2017 by the American Association for the Study of Liver Diseases.
Figures


Comment in
-
Is mIndy a mediator of energy metabolism reprogramming in hepatocellular carcinoma induced by interleukin-6/signal transducer and activator of transcription 3 signaling?Hepatology. 2018 Jan;67(1):451-452. doi: 10.1002/hep.29592. Hepatology. 2018. PMID: 29023910 No abstract available.
-
Reply.Hepatology. 2018 Jan;67(1):452-453. doi: 10.1002/hep.29591. Hepatology. 2018. PMID: 29024037 No abstract available.
Similar articles
-
Prevention of diet-induced hepatic steatosis and hepatic insulin resistance by second generation antisense oligonucleotides targeted to the longevity gene mIndy (Slc13a5).Aging (Albany NY). 2015 Dec;7(12):1086-93. doi: 10.18632/aging.100854. Aging (Albany NY). 2015. PMID: 26647160 Free PMC article.
-
Inhibition of citrate cotransporter Slc13a5/mINDY by RNAi improves hepatic insulin sensitivity and prevents diet-induced non-alcoholic fatty liver disease in mice.Mol Metab. 2016 Aug 13;5(11):1072-1082. doi: 10.1016/j.molmet.2016.08.004. eCollection 2016 Nov. Mol Metab. 2016. PMID: 27818933 Free PMC article.
-
Arylhydrocarbon receptor-dependent mIndy (Slc13a5) induction as possible contributor to benzo[a]pyrene-induced lipid accumulation in hepatocytes.Toxicology. 2015 Nov 4;337:1-9. doi: 10.1016/j.tox.2015.08.007. Epub 2015 Aug 21. Toxicology. 2015. PMID: 26303333
-
The longevity gene INDY (I'm Not Dead Yet) in metabolic control: Potential as pharmacological target.Pharmacol Ther. 2018 May;185:1-11. doi: 10.1016/j.pharmthera.2017.10.003. Epub 2017 Oct 5. Pharmacol Ther. 2018. PMID: 28987323 Review.
-
INDY-A New Link to Metabolic Regulation in Animals and Humans.Front Genet. 2017 May 24;8:66. doi: 10.3389/fgene.2017.00066. eCollection 2017. Front Genet. 2017. PMID: 28596784 Free PMC article. Review.
Cited by
-
Plasma Membrane Na⁺-Coupled Citrate Transporter (SLC13A5) and Neonatal Epileptic Encephalopathy.Molecules. 2017 Feb 28;22(3):378. doi: 10.3390/molecules22030378. Molecules. 2017. PMID: 28264506 Free PMC article. Review.
-
Analysis of naturally occurring mutations in the human uptake transporter NaCT important for bone and brain development and energy metabolism.Sci Rep. 2018 Jul 27;8(1):11330. doi: 10.1038/s41598-018-29547-8. Sci Rep. 2018. PMID: 30054523 Free PMC article.
-
Citric Acid: A Multifunctional Pharmaceutical Excipient.Pharmaceutics. 2022 Apr 30;14(5):972. doi: 10.3390/pharmaceutics14050972. Pharmaceutics. 2022. PMID: 35631557 Free PMC article. Review.
-
Piperine alleviates nonalcoholic steatohepatitis by inhibiting NF-κB-mediated hepatocyte pyroptosis.PLoS One. 2024 Mar 28;19(3):e0301133. doi: 10.1371/journal.pone.0301133. eCollection 2024. PLoS One. 2024. PMID: 38547097 Free PMC article.
-
INDY-From Flies to Worms, Mice, Rats, Non-Human Primates, and Humans.Front Aging. 2021 Dec 23;2:782162. doi: 10.3389/fragi.2021.782162. eCollection 2021. Front Aging. 2021. PMID: 35822025 Free PMC article. Review.
References
-
- Rogina B, Reenan RA, Nilsen SP, Helfand SL. Extended life-span conferred by cotransporter gene mutations in Drosophila. Science. 2000;290:2137–2140. - PubMed
MeSH terms
Substances
Grants and funding
- R01 DK049230/DK/NIDDK NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- U24 DK059635/DK/NIDDK NIH HHS/United States
- R01 AG024353/AG/NIA NIH HHS/United States
- RF1 AG024353/AG/NIA NIH HHS/United States
- P30 DK045735/DK/NIDDK NIH HHS/United States
- P30 DK034989/DK/NIDDK NIH HHS/United States
- R01 AG016667/AG/NIA NIH HHS/United States
- Z99 AG999999/ImNIH/Intramural NIH HHS/United States
- R01 AG025277/AG/NIA NIH HHS/United States
- R37 AG016667/AG/NIA NIH HHS/United States
- R01 DK040936/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

