HIV antibodies for treatment of HIV infection
- PMID: 28133794
- PMCID: PMC5556378
- DOI: 10.1111/imr.12506
HIV antibodies for treatment of HIV infection
Abstract
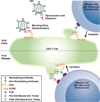
The bar is high to improve on current combination antiretroviral therapy (ART), now highly effective, safe, and simple. However, antibodies that bind the HIV envelope are able to uniquely target the virus as it seeks to enter new target cells, or as it is expressed from previously infected cells. Furthermore, the use of antibodies against HIV as a therapeutic may offer advantages. Antibodies can have long half-lives, and are being considered as partners for long-acting antiretrovirals for use in therapy or prevention of HIV infection. Early studies in animal models and in clinical trials suggest that such antibodies can have antiviral activity but, as with small-molecule antiretrovirals, the issues of viral escape and resistance will have to be addressed. Most promising, however, are the unique properties of anti-HIV antibodies: the potential ability to opsonize viral particles, to direct antibody-dependent cellular cytotoxicity (ADCC) against actively infected cells, and ultimately the ability to direct the clearance of HIV-infected cells by effector cells of the immune system. These distinctive activities suggest that HIV antibodies and their derivatives may play an important role in the next frontier of HIV therapeutics, the effort to develop treatments that could lead to an HIV cure.
Keywords: ADCC; HIV; cure; entry inhibition; monoclonal antibodies.
Published 2017. This article is a U.S. Government work and is in the public domain in the USA.
Conflict of interest statement
Conflict of Interest statement:
DM and RK have no conflicts of interest to disclose.
Figures
Similar articles
-
Evolution of Antibodies to Native Trimeric Envelope and Their Fc-Dependent Functions in Untreated and Treated Primary HIV Infection.J Virol. 2021 Nov 23;95(24):e0162521. doi: 10.1128/JVI.01625-21. Epub 2021 Sep 29. J Virol. 2021. PMID: 34586863 Free PMC article.
-
The Early Antibody-Dependent Cell-Mediated Cytotoxicity Response Is Associated With Lower Viral Set Point in Individuals With Primary HIV Infection.Front Immunol. 2018 Oct 9;9:2322. doi: 10.3389/fimmu.2018.02322. eCollection 2018. Front Immunol. 2018. PMID: 30356637 Free PMC article.
-
Anti-R7V antibodies as therapeutics for HIV-infected patients in failure of HAART.Curr Opin Biotechnol. 2002 Dec;13(6):621-4. doi: 10.1016/s0958-1669(02)00381-6. Curr Opin Biotechnol. 2002. PMID: 12482524 Review.
-
HIV-specific CD4-induced Antibodies Mediate Broad and Potent Antibody-dependent Cellular Cytotoxicity Activity and Are Commonly Detected in Plasma From HIV-infected humans.EBioMedicine. 2015 Oct;2(10):1464-77. doi: 10.1016/j.ebiom.2015.09.001. EBioMedicine. 2015. PMID: 26629541 Free PMC article.
-
Epitope specificity of human immunodeficiency virus-1 antibody dependent cellular cytotoxicity [ADCC] responses.Curr HIV Res. 2013 Jul;11(5):378-87. doi: 10.2174/1570162x113116660059. Curr HIV Res. 2013. PMID: 24191939 Free PMC article. Review.
Cited by
-
IgG1-b12-HIV-gp120 Interface in Solution: A Computational Study.J Chem Inf Model. 2022 Jan 24;62(2):359-371. doi: 10.1021/acs.jcim.1c01143. Epub 2021 Dec 31. J Chem Inf Model. 2022. PMID: 34971312 Free PMC article.
-
Implementation of a three-tiered approach to identify and characterize anti-drug antibodies raised against HIV-specific broadly neutralizing antibodies.J Immunol Methods. 2020 Apr;479:112764. doi: 10.1016/j.jim.2020.112764. Epub 2020 Feb 15. J Immunol Methods. 2020. PMID: 32070674 Free PMC article.
-
Trispecific broadly neutralizing HIV antibodies mediate potent SHIV protection in macaques.Science. 2017 Oct 6;358(6359):85-90. doi: 10.1126/science.aan8630. Epub 2017 Sep 20. Science. 2017. PMID: 28931639 Free PMC article.
-
Considerations for designing and implementing combination HIV cure trials: findings from a qualitative in-depth interview study in the United States.AIDS Res Ther. 2021 Oct 18;18(1):75. doi: 10.1186/s12981-021-00401-8. AIDS Res Ther. 2021. PMID: 34663375 Free PMC article.
-
Drug Resistance Prediction Using Deep Learning Techniques on HIV-1 Sequence Data.Viruses. 2020 May 19;12(5):560. doi: 10.3390/v12050560. Viruses. 2020. PMID: 32438586 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

