The HIV-1 envelope glycoprotein structure: nailing down a moving target
- PMID: 28133813
- PMCID: PMC5300090
- DOI: 10.1111/imr.12507
The HIV-1 envelope glycoprotein structure: nailing down a moving target
Abstract
Structure determination of the HIV-1 envelope glycoprotein (Env) presented a number of challenges, but several high-resolution structures have now become available. In 2013, cryo-EM and x-ray structures of soluble, cleaved SOSIP Env trimers from the clade A BG505 strain provided the first glimpses into the Env trimer fold as well as more the variable regions. A recent cryo-EM structure of a native full-length trimer without any stabilizing mutations had the same core structure, but revealed new insights and features. A more comprehensive and higher resolution understanding of the glycan shield has also emerged, enabling a more complete representation of the Env glycoprotein structure. Complexes of Env trimers with broadly neutralizing antibodies have surprisingly illustrated that most of the Env surface can be targeted in natural infection and that the neutralizing epitopes are almost all composed of both peptide and glycan components. These structures have also provided further evidence of the inherent plasticity of Env and how antibodies can exploit this flexibility by perturbing or even stabilizing the trimer to facilitate neutralization. These breakthroughs have stimulated further design and stabilization of Env trimers as well as other platforms to generate trimers that now span multiple subtypes. These Env trimers when used as immunogens, have led to the first vaccine-induced neutralizing antibodies for structural and functional analyses.
Keywords: HIV envelope structure; cryo-electron microscopy; epitope mapping; glycan shield; structure-based vaccine design; x-ray crystallography.
© 2017 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
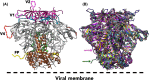



References
-
- Brand CM, Skehel JJ. Crystalline antigen from the influenza virus envelope. Nat New Biol. 1972;238:145–147. - PubMed
-
- Wiley DC, Skehel JJ. Crystallization and x‐ray diffraction studies on the haemagglutinin glycoprotein from the membrane of influenza virus. J Mol Biol. 1977;112:343–347. - PubMed
-
- Wrigley NG, Laver WG, Downie JC. Binding of antibodies to isolated haemagglutinin and neuraminidase molecules of influenza virus observed in the electron microscope. J Mol Biol. 1977;109:405–421. - PubMed
-
- Laver WG, Valentine RC. Morphology of the isolated hemagglutinin and neuraminidase subunits of influenza virus. Virology. 1969;38:105–119. - PubMed
-
- Wrigley NG, Skehel JJ, Charlwood PA, Brand CM. The size and shape of influenza virus neuraminidase. Virology. 1973;51:525–529. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

