Clone and functional analysis of Seryl-tRNA synthetase and Tyrosyl-tRNA synthetase from silkworm, Bombyx mori
- PMID: 28134300
- PMCID: PMC5278501
- DOI: 10.1038/srep41563
Clone and functional analysis of Seryl-tRNA synthetase and Tyrosyl-tRNA synthetase from silkworm, Bombyx mori
Abstract
Aminoacyl-tRNA synthetases are the key enzymes for protein synthesis. Glycine, alanine, serine and tyrosine are the major amino acids composing fibroin of silkworm. Among them, the genes of alanyl-tRNA synthetase (AlaRS) and glycyl-tRNA synthetase (GlyRS) have been cloned. In this study, the seryl-tRNA synthetase (SerRS) and tyrosyl-tRNA synthetase (TyrRS) genes from silkworm were cloned. Their full length are 1709 bp and 1868 bp and contain open reading frame (ORF) of 1485 bp and 1575 bp, respectively. RT-PCR examination showed that the transcription levels of SerRS, TyrRS, AlaRS and GlyRS are significantly higher in silk gland than in other tissues. In addition, their transcription levels are much higher in middle and posterior silk gland than in anterior silk gland. Moreover, treatment of silkworms with phoxim, an inhibitor of silk protein synthesis, but not TiO2 NP, an enhancer of silk protein synthesis, significantly reduced the transcription levels of aaRS and content of free amino acids in posterior silk gland, therefore affecting silk protein synthesis, which may be the mechanism of phoxim-silking disorders. Furthermore, low concentration of TiO2 NPs showed no effect on the transcription of aaRS and content of free amino acids, suggesting that TiO2 NPs promotes silk protein synthesis possibly by increasing the activity of fibroin synthase in silkworm.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
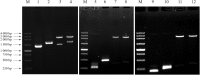

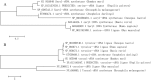


Similar articles
-
Effects of Titanium Dioxide Nanoparticles on the Synthesis of Fibroin in Silkworm (Bombyx mori).Biol Trace Elem Res. 2015 Aug;166(2):225-35. doi: 10.1007/s12011-015-0258-y. Epub 2015 Feb 11. Biol Trace Elem Res. 2015. PMID: 25876086
-
Control of the levels of alanyl-, glycyl-, and seryl-tRNA synthetases in the silkgland of Bombyx mori.Dev Biol. 1988 Oct;129(2):350-7. doi: 10.1016/0012-1606(88)90382-x. Dev Biol. 1988. PMID: 3417042
-
The biosynthesis of transfer RNA in insects. I. Increase of amino acid acceptor activity of specific tRNA's utilized for silk protein biosynthesis in the silk gland of Bombyx mori.J Biochem. 1975 Aug;78(2):391-400. doi: 10.1093/oxfordjournals.jbchem.a130919. J Biochem. 1975. PMID: 1228175
-
Glycyl-tRNA synthetase.Biol Chem Hoppe Seyler. 1996 Jun;377(6):343-56. Biol Chem Hoppe Seyler. 1996. PMID: 8839980 Review.
-
Non-canonical functions of human cytoplasmic tyrosyl-, tryptophanyl- and other aminoacyl-tRNA synthetases.Enzymes. 2020;48:207-242. doi: 10.1016/bs.enz.2020.04.001. Epub 2020 Jun 12. Enzymes. 2020. PMID: 33837705 Review.
Cited by
-
Metabonomic Analysis of Silkworm Midgut Reveals Differences between the Physiological Effects of an Artificial and Mulberry Leaf Diet.Insects. 2023 Mar 31;14(4):347. doi: 10.3390/insects14040347. Insects. 2023. PMID: 37103160 Free PMC article.
-
Chronic Low-Dose Phoxim Exposure Impairs Silk Production in Bombyx mori L. (Lepidoptera: Bombycidae) by Disrupting Juvenile Hormone Signaling-Mediated Fibroin Synthesis.Toxics. 2025 May 23;13(6):427. doi: 10.3390/toxics13060427. Toxics. 2025. PMID: 40559901 Free PMC article.
References
-
- Zhang Y. Q. Applications of natural silk protein sericin in biomaterials. Biotechnol. Adv. 20, 91–100 (2002). - PubMed
-
- Zhou C. Z. et al.. Silk fibroin: structural implications of a remarkable amino acid sequence. Proteins: Struct., Funct., Bioinf. 44, 119–122 (2001). - PubMed
-
- Rock F. L. et al.. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science. 316, 1759–1761 (2007). - PubMed
-
- Cusack S., Berthet-Colominas C., Härtlein M., Nassar N. & Leberman R. A second class of synthetase structure revealed by X-ray analysis of Escherichia coli seryl-tRNA synthetase at 2.5 Å. Nature. 347, 249–255 (1990). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

