Glucose Outcomes with the In-Home Use of a Hybrid Closed-Loop Insulin Delivery System in Adolescents and Adults with Type 1 Diabetes
- PMID: 28134564
- PMCID: PMC5359676
- DOI: 10.1089/dia.2016.0421
Glucose Outcomes with the In-Home Use of a Hybrid Closed-Loop Insulin Delivery System in Adolescents and Adults with Type 1 Diabetes
Abstract
Background: The safety and effectiveness of the in-home use of a hybrid closed-loop (HCL) system that automatically increases, decreases, and suspends insulin delivery in response to continuous glucose monitoring were investigated.
Methods: Adolescents (n = 30, ages 14-21 years) and adults (n = 94, ages 22-75 years) with type 1 diabetes participated in a multicenter (nine sites in the United States, one site in Israel) pivotal trial. The Medtronic MiniMed® 670G system was used during a 2-week run-in phase without HCL control, or Auto Mode, enabled (Manual Mode) and, thereafter, with Auto Mode enabled during a 3-month study phase. A supervised hotel stay (6 days/5 nights) that included a 24-h frequent blood sample testing with a reference measurement (i-STAT) occurred during the study phase.
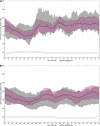
Results: Adolescents (mean ± standard deviation [SD] 16.5 ± 2.29 years of age and 7.7 ± 4.15 years of diabetes) used the system for a median 75.8% (interquartile range [IQR] 68.0%-88.4%) of the time (2977 patient-days). Adults (mean ± SD 44.6 ± 12.79 years of age and 26.4 ± 12.43 years of diabetes) used the system for a median 88.0% (IQR 77.6%-92.7%) of the time (9412 patient-days). From baseline run-in to the end of study phase, adolescent and adult HbA1c levels decreased from 7.7% ± 0.8% to 7.1% ± 0.6% (P < 0.001) and from 7.3% ± 0.9% to 6.8% ± 0.6% (P < 0.001, Wilcoxon signed-rank test), respectively. The proportion of overall in-target (71-180 mg/dL) sensor glucose (SG) values increased from 60.4% ± 10.9% to 67.2% ± 8.2% (P < 0.001) in adolescents and from 68.8% ± 11.9% to 73.8% ± 8.4% (P < 0.001) in adults. During the hotel stay, the proportion of in-target i-STAT® blood glucose values was 67.4% ± 27.7% compared to SG values of 72.0% ± 11.6% for adolescents and 74.2% ± 17.5% compared to 76.9% ± 8.3% for adults. There were no severe hypoglycemic or diabetic ketoacidosis events in either cohort.
Conclusions: HCL therapy was safe during in-home use by adolescents and adults and the study phase demonstrated increased time in target, and reductions in HbA1c, hyperglycemia and hypoglycemia, compared to baseline.
Trial registration: Clinicaltrials.gov identifier: NCT02463097.
Keywords: Continuous glucose monitoring; Hybrid closed loop; Hyperglycemia; Hypoglycemia; Insulin pump; Sensor; Type 1 diabetes.
Conflict of interest statement
The following authors: Drs. Garg, Weinzimer, Buckingham, Bode, Bailey, Brazg, Ilany, Slover, Anderson, and Bergenstal served as principal investigators for this study and received compensation and research support from Medtronic. Dr. Tamborlane served as a consultant in the study and received compensation from Medtronic. Drs. Grosman, Roy, Cordero, Shin, Lee, and Kaufman, employees of Medtronic, participated in data analysis and/or critical review of the manuscript.
Figures
References
-
- Miller KM, Foster NC, Beck RW, et al. : Current state of type 1 diabetes treatment in the U.S.: Updated data from the T1D Exchange clinic registry. Diabetes Care 2015;38:971–978 - PubMed
-
- Weinzimer SA, Steil GM, Swan KL, et al. : Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care 2008;31:934–939 - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

