From Immunologically Archaic to Neoteric Glycovaccines
- PMID: 28134792
- PMCID: PMC5371740
- DOI: 10.3390/vaccines5010004
From Immunologically Archaic to Neoteric Glycovaccines
Abstract
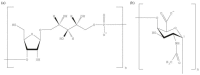
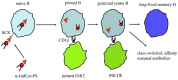
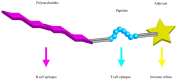
Polysaccharides (PS) are present in the outermost surface of bacteria and readily come in contact with immune cells. They interact with specific antibodies, which in turn confer protection from infections. Vaccines with PS from pneumococci, meningococci, Haemophilus influenzae type b, and Salmonella typhi may be protective, although with the important constraint of failing to generate permanent immunological memory. This limitation has in part been circumvented by conjugating glycovaccines to proteins that stimulate T helper cells and facilitate the establishment of immunological memory. Currently, protection evoked by conjugated PS vaccines lasts for a few years. The same approach failed with PS from staphylococci, Streptococcus agalactiae, and Klebsiella. All those germs cause severe infections in humans and often develop resistance to antibiotic therapy. Thereby, prevention is of increasing importance to better control outbreaks. As only 23 of more than 90 pneumococcal serotypes and 4 of 13 clinically relevant Neisseria meningitidis serogroups are covered by available vaccines there is still tremendous clinical need for PS vaccines. This review focuses on glycovaccines and the immunological mechanisms for their success or failure. We discuss recent advances that may facilitate generation of high affinity anti-PS antibodies and confer specific immunity and long-lasting protection.
Keywords: T cell help; conjugate; immunosenescence; polysaccharide; serotype; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

