Trm112, a Protein Activator of Methyltransferases Modifying Actors of the Eukaryotic Translational Apparatus
- PMID: 28134793
- PMCID: PMC5372719
- DOI: 10.3390/biom7010007
Trm112, a Protein Activator of Methyltransferases Modifying Actors of the Eukaryotic Translational Apparatus
Abstract
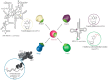
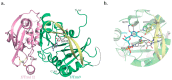
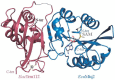
Post-transcriptional and post-translational modifications are very important for the control and optimal efficiency of messenger RNA (mRNA) translation. Among these, methylation is the most widespread modification, as it is found in all domains of life. These methyl groups can be grafted either on nucleic acids (transfer RNA (tRNA), ribosomal RNA (rRNA), mRNA, etc.) or on protein translation factors. This review focuses on Trm112, a small protein interacting with and activating at least four different eukaryotic methyltransferase (MTase) enzymes modifying factors involved in translation. The Trm112-Trm9 and Trm112-Trm11 complexes modify tRNAs, while the Trm112-Mtq2 complex targets translation termination factor eRF1, which is a tRNA mimic. The last complex formed between Trm112 and Bud23 proteins modifies 18S rRNA and participates in the 40S biogenesis pathway. In this review, we present the functions of these eukaryotic Trm112-MTase complexes, the molecular bases responsible for complex formation and substrate recognition, as well as their implications in human diseases. Moreover, as Trm112 orthologs are found in bacterial and archaeal genomes, the conservation of this Trm112 network beyond eukaryotic organisms is also discussed.
Keywords: RNA modifying enzyme; S-adenosyl-l-methionine; methyltransferase; post-transcriptional modification; translation.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; nor in the decision to publish the results.
Figures




Similar articles
-
Activation mode of the eukaryotic m2G10 tRNA methyltransferase Trm11 by its partner protein Trm112.Nucleic Acids Res. 2017 Feb 28;45(4):1971-1982. doi: 10.1093/nar/gkw1271. Nucleic Acids Res. 2017. PMID: 27986851 Free PMC article.
-
Insights into molecular plasticity in protein complexes from Trm9-Trm112 tRNA modifying enzyme crystal structure.Nucleic Acids Res. 2015 Dec 15;43(22):10989-1002. doi: 10.1093/nar/gkv1009. Epub 2015 Oct 4. Nucleic Acids Res. 2015. PMID: 26438534 Free PMC article.
-
Structural and functional studies of Bud23-Trm112 reveal 18S rRNA N7-G1575 methylation occurs on late 40S precursor ribosomes.Proc Natl Acad Sci U S A. 2014 Dec 23;111(51):E5518-26. doi: 10.1073/pnas.1413089111. Epub 2014 Dec 8. Proc Natl Acad Sci U S A. 2014. PMID: 25489090 Free PMC article.
-
[The Common Partner of Several Methyltransferases Modifying the Components of The Eukaryotic Translation Apparatus].Mol Biol (Mosk). 2018 Nov-Dec;52(6):975-983. doi: 10.1134/S0026898418060174. Mol Biol (Mosk). 2018. PMID: 30633240 Review. Russian.
-
Two-subunit enzymes involved in eukaryotic post-transcriptional tRNA modification.RNA Biol. 2014;11(12):1608-18. doi: 10.1080/15476286.2015.1008360. RNA Biol. 2014. PMID: 25625329 Free PMC article. Review.
Cited by
-
To be or not to be modified: Miscellaneous aspects influencing nucleotide modifications in tRNAs.IUBMB Life. 2019 Aug;71(8):1126-1140. doi: 10.1002/iub.2041. Epub 2019 Apr 1. IUBMB Life. 2019. PMID: 30932315 Free PMC article. Review.
-
Pan-Cancer Analysis Reveals the Relation between TRMT112 and Tumor Microenvironment.J Oncol. 2022 Aug 30;2022:1445932. doi: 10.1155/2022/1445932. eCollection 2022. J Oncol. 2022. PMID: 36081672 Free PMC article.
-
Structural and functional insights into Archaeoglobus fulgidus m2G10 tRNA methyltransferase Trm11 and its Trm112 activator.Nucleic Acids Res. 2020 Nov 4;48(19):11068-11082. doi: 10.1093/nar/gkaa830. Nucleic Acids Res. 2020. PMID: 33035335 Free PMC article.
-
Overexpression of 18S rRNA methyltransferase CrBUD23 enhances biomass and lutein content in Chlamydomonas reinhardtii.Front Bioeng Biotechnol. 2023 Feb 3;11:1102098. doi: 10.3389/fbioe.2023.1102098. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 36815903 Free PMC article.
-
Transfer RNA Modification Enzymes with a Thiouridine Synthetase, Methyltransferase and Pseudouridine Synthase (THUMP) Domain and the Nucleosides They Produce in tRNA.Genes (Basel). 2023 Jan 31;14(2):382. doi: 10.3390/genes14020382. Genes (Basel). 2023. PMID: 36833309 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

