Acidosis Activates Endoplasmic Reticulum Stress Pathways through GPR4 in Human Vascular Endothelial Cells
- PMID: 28134810
- PMCID: PMC5343814
- DOI: 10.3390/ijms18020278
Acidosis Activates Endoplasmic Reticulum Stress Pathways through GPR4 in Human Vascular Endothelial Cells
Abstract
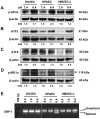
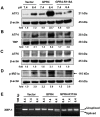
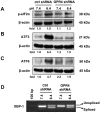
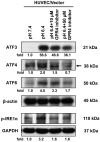
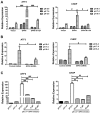
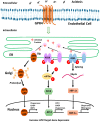
Acidosis commonly exists in the tissue microenvironment of various pathophysiological conditions such as tumors, inflammation, ischemia, metabolic disease, and respiratory disease. For instance, the tumor microenvironment is characterized by acidosis and hypoxia due to tumor heterogeneity, aerobic glycolysis (the "Warburg effect"), and the defective vasculature that cannot efficiently deliver oxygen and nutrients or remove metabolic acid byproduct. How the acidic microenvironment affects the function of blood vessels, however, is not well defined. GPR4 (G protein-coupled receptor 4) is a member of the proton-sensing G protein-coupled receptors and it has high expression in endothelial cells (ECs). We have previously reported that acidosis induces a broad inflammatory response in ECs. Acidosis also increases the expression of several endoplasmic reticulum (ER) stress response genes such as CHOP (C/EBP homologous protein) and ATF3 (activating transcription factor 3). In the current study, we have examined acidosis/GPR4- induced ER stress pathways in human umbilical vein endothelial cells (HUVEC) and other types of ECs. All three arms of the ER stress/unfolded protein response (UPR) pathways were activated by acidosis in ECs as an increased expression of phosphorylated eIF2α (eukaryotic initiation factor 2α), phosphorylated IRE1α (inositol-requiring enzyme 1α), and cleaved ATF6 upon acidic pH treatment was observed. The expression of other downstream mediators of the UPR, such as ATF4, ATF3, and spliced XBP-1 (X box-binding protein 1), was also induced by acidosis. Through genetic and pharmacological approaches to modulate the expression level or activity of GPR4 in HUVEC, we found that GPR4 plays an important role in mediating the ER stress response induced by acidosis. As ER stress/UPR can cause inflammation and cell apoptosis, acidosis/GPR4-induced ER stress pathways in ECs may regulate vascular growth and inflammatory response in the acidic microenvironment.
Keywords: G protein-coupled receptor 4 (GPR4); acidosis; blood vessel; endoplasmic reticulum (ER) stress; endothelial cell (EC); pH; tissue microenvironment; unfolded protein response (UPR).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
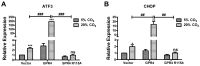
References
-
- Sanderlin E.J., Justus C.R., Krewson E.A., Yang L.V. Emerging roles for the pH-sensing G protein-coupled receptors in response to acidotic stress. Cell Health Cytoskelet. 2015;7:99–109.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

