Cytotoxic Compounds Derived from Marine Sponges. A Review (2010-2012)
- PMID: 28134844
- PMCID: PMC6155849
- DOI: 10.3390/molecules22020208
Cytotoxic Compounds Derived from Marine Sponges. A Review (2010-2012)
Abstract
Abstract: This extensive review covers research published between 2010 and 2012 regarding new compounds derived from marine sponges, including 62 species from 60 genera belonging to 33 families and 13 orders of the Demospongia class (Porifera). The emphasis is on the cytotoxic activity that bioactive metabolites from sponges may have on cancer cell lines. At least 197 novel chemical structures from 337 compounds isolated have been found to support this work. Details on the source and taxonomy of the sponges, their geographical occurrence, and a range of chemical structures are presented. The compounds discovered from the reviewed marine sponges fall into mainly four chemical classes: terpenoids (41.9%), alkaloids (26.2%), macrolides (8.9%) and peptides (6.3%) which, along with polyketides, sterols, and others show a range of biological activities. The key sponge orders studied in the reviewed research were Dictyoceratida, Haplosclerida, Tetractinellida, Poecilosclerida, and Agelasida. Petrosia, Haliclona (Haplosclerida), Rhabdastrella (Tetractinellida), Coscinoderma and Hyppospongia (Dictyioceratida), were found to be the most promising genera because of their capacity for producing new bioactive compounds. Several of the new compounds and their synthetic analogues have shown in vitro cytotoxic and pro-apoptotic activities against various tumor/cancer cell lines, and some of them will undergo further in vivo evaluation.
Keywords: Keywords: porifera; bioactive molecules; cancer cell lines; cytotoxicity; marine sponges; pharmacology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
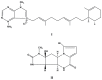
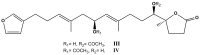
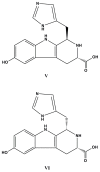

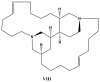



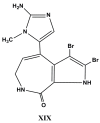
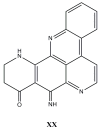

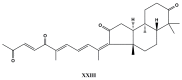
References
-
- Jones A.C., Blum J.E., Pawlik J.R. Testing for defensive synergy in Caribbean sponges: Bad taste or glass spicules? J. Exp. Mar. Biol. Ecol. 2005;322:67–81. doi: 10.1016/j.jembe.2005.02.009. - DOI
-
- Ribeiro S.M., Cassiano K.M., Cavalcanti D.N., Teixeira V.L., Pereira R.C. Isolated and synergistic effects of chemical and structural defenses of two species of Tethya (Porifera: Demospongiae) J. Sea Res. 2012;68:57–62. doi: 10.1016/j.seares.2011.12.002. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

