The IgM receptor FcμR limits tonic BCR signaling by regulating expression of the IgM BCR
- PMID: 28135254
- PMCID: PMC5310993
- DOI: 10.1038/ni.3677
The IgM receptor FcμR limits tonic BCR signaling by regulating expression of the IgM BCR
Abstract
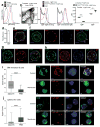
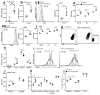
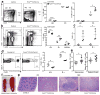
The FcμR receptor for the crystallizable fragment (Fc) of immunoglobulin M (IgM) can function as a cell-surface receptor for secreted IgM on a variety of cell types. We found here that FcμR was also expressed in the trans-Golgi network of developing B cells, where it constrained transport of the IgM-isotype BCR (IgM-BCR) but not of the IgD-isotype BCR (IgD-BCR). In the absence of FcμR, the surface expression of IgM-BCR was increased, which resulted in enhanced tonic BCR signaling. B-cell-specific deficiency in FcμR enhanced the spontaneous differentiation of B-1 cells, which resulted in increased serum concentrations of natural IgM and dysregulated homeostasis of B-2 cells; this caused the spontaneous formation of germinal centers, increased titers of serum autoantibodies and excessive accumulation of B cells. Thus, FcμR serves as a critical regulator of B cell biology by constraining the transport and cell-surface expression of IgM-BCR.
Conflict of interest statement
Competing Financial Interests
The authors declare that no conflict of interest exists.
Figures




References
-
- Nguyen XH, et al. Toso regulates the balance between apoptotic and nonapoptotic death receptor signaling by facilitating RIP1 ubiquitination. Blood. 2011;118:598–608. - PubMed
-
- Nguyen XH, et al. Antiapoptotic function of Toso (Faim3) in death receptor signaling. Blood. 2012;119:1790–1791. - PubMed
-
- Hitoshi Y, et al. Toso, a cell surface, specific regulator of Fas-induced apoptosis in T cells. Immunity. 1998;8:461–471. - PubMed
-
- Ouchida R, Mori H, Ohno H, Wang JY. FcmuR (Toso/Faim3) is not an inhibitor of Fas-mediated cell death in mouse T and B cells. Blood. 2013;121:2368–2370. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

