Human Cord Blood and Bone Marrow CD34+ Cells Generate Macrophages That Support Erythroid Islands
- PMID: 28135323
- PMCID: PMC5279789
- DOI: 10.1371/journal.pone.0171096
Human Cord Blood and Bone Marrow CD34+ Cells Generate Macrophages That Support Erythroid Islands
Abstract
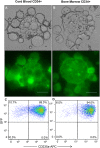
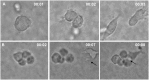
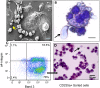
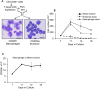
Recently, we developed a small molecule responsive hyperactive Mpl-based Cell Growth Switch (CGS) that drives erythropoiesis associated with macrophages in the absence of exogenous cytokines. Here, we compare the physical, cellular and molecular interaction between the macrophages and erythroid cells in CGS expanded CD34+ cells harvested from cord blood, marrow or G-CSF-mobilized peripheral blood. Results indicated that macrophage based erythroid islands could be generated from cord blood and marrow CD34+ cells but not from G-CSF-mobilized CD34+ cells. Additional studies suggest that the deficiency resides with the G-CSF-mobilized CD34+ derived monocytes. Gene expression and proteomics studies of the in vitro generated erythroid islands detected the expression of erythroblast macrophage protein (EMP), intercellular adhesion molecule 4 (ICAM-4), CD163 and DNASE2. 78% of the erythroblasts in contact with macrophages reached the pre reticulocyte orthochromatic stage of differentiation within 14 days of culture. The addition of conditioned medium from cultures of CD146+ marrow fibroblasts resulted in a 700-fold increase in total cell number and a 90-fold increase in erythroid cell number. This novel CD34+ cell derived erythroid island may serve as a platform to explore the molecular basis of red cell maturation and production under normal, stress and pathological conditions.
Conflict of interest statement
C.A.B. is named as an inventor on a patent for the CGS technology licensed to Ariad Pharmaceuticals, Inc. through the University of Washington. All other authors declare no competing financial interests. We would like to bring to your attention that, all authors declare no competing interests as the patent related to one author described in the original submission have expired.
Figures


References
-
- Bessis M. Erythroblastic island, functional unity of bone marrow [French]. Revue d'Hématologie. 1958;13(1):11-Aug. - PubMed
-
- Allen TD, Dexter TM. Ultrastructural aspects of erythropoietic differentiation in long-term bone marrow culture. Differentiation. 1982;21(2):86–94. - PubMed
-
- Hanspal M, Smockova Y, Uong Q. Molecular identification and functional characterization of a novel protein that mediates the attachment of erythroblasts to macrophages. Blood. 1998;92(8):2940–50. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

