Blocking two-component signalling enhances Candida albicans virulence and reveals adaptive mechanisms that counteract sustained SAPK activation
- PMID: 28135328
- PMCID: PMC5300278
- DOI: 10.1371/journal.ppat.1006131
Blocking two-component signalling enhances Candida albicans virulence and reveals adaptive mechanisms that counteract sustained SAPK activation
Abstract
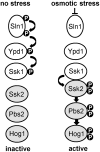
The Ypd1 phosphorelay protein is a central constituent of fungal two-component signal transduction pathways. Inhibition of Ypd1 in Saccharomyces cerevisiae and Cryptococcus neoformans is lethal due to the sustained activation of the 'p38-related' Hog1 stress-activated protein kinase (SAPK). As two-component signalling proteins are not found in animals, Ypd1 is considered to be a prime antifungal target. However, a major fungal pathogen of humans, Candida albicans, can survive the concomitant sustained activation of Hog1 that occurs in cells lacking YPD1. Here we show that the sustained activation of Hog1 upon Ypd1 loss is mediated through the Ssk1 response regulator. Moreover, we present evidence that C. albicans survives SAPK activation in the short-term, following Ypd1 loss, by triggering the induction of protein tyrosine phosphatase-encoding genes which prevent the accumulation of lethal levels of phosphorylated Hog1. In addition, our studies reveal an unpredicted, reversible, mechanism that acts to substantially reduce the levels of phosphorylated Hog1 in ypd1Δ cells following long-term sustained SAPK activation. Indeed, over time, ypd1Δ cells become phenotypically indistinguishable from wild-type cells. Importantly, we also find that drug-induced down-regulation of YPD1 expression actually enhances the virulence of C. albicans in two distinct animal infection models. Investigating the underlying causes of this increased virulence, revealed that drug-mediated repression of YPD1 expression promotes hyphal growth both within murine kidneys, and following phagocytosis, thus increasing the efficacy by which C. albicans kills macrophages. Taken together, these findings challenge the targeting of Ypd1 proteins as a general antifungal strategy and reveal novel cellular adaptation mechanisms to sustained SAPK activation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
MeSH terms
Substances
Grants and funding
- BB/K016393/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 097377/WT_/Wellcome Trust/United Kingdom
- 086048/WT_/Wellcome Trust/United Kingdom
- MR/N006364/1/MRC_/Medical Research Council/United Kingdom
- BB/K017365/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

