PR-independent neurosteroid regulation of α2-GABA-A receptors in the hippocampus subfields
- PMID: 28137424
- PMCID: PMC5367384
- DOI: 10.1016/j.brainres.2017.01.030
PR-independent neurosteroid regulation of α2-GABA-A receptors in the hippocampus subfields
Abstract
Progesterone (P) binding to the intracellular progesterone receptors (PRs) plays a key role in epilepsy via modulation of GABA-A receptor plasticity in the brain. This is thought to occur via conversion of P to neurosteroids such as allopregnanolone, an allosteric modulator of GABA-A receptors. In the female brain, the composition of GABA-A receptors is not static and undergoes dynamic spatial changes in response to fluctuations in P and neurosteroid levels. Synaptic α2-containing GABA-A receptors contribute to phasic neuronal excitability and seizure susceptibility. However, the mechanisms underlying α2-subunit plasticity remain unclear. Here, we utilized the neurosteroid synthesis inhibitor finasteride and PR knockout mice to investigate the role of PRs in α2-subunit in the hippocampus. α2-Subunit expression was significantly upregulated during the high-P state of diestrous stage and with P treatment in wildtype and PR knockout mice. In contrast, there was no change in α2-subunit expression when metabolism of P into neurosteroids was blocked by finasteride in both genotypes. These findings suggest that ovarian cycle-related P and neurosteroids regulate α2-GABA-A receptor expression in the hippocampus via a non-PR pathway, which may be relevant to menstrual-cycle related brain conditions.
Keywords: Epilepsy; GABA receptor; Neurosteroid; Perimenstrual; Progesterone; α2-Subunit.
Copyright © 2017 Elsevier B.V. All rights reserved.
Conflict of interest statement
None.
Figures

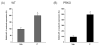
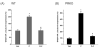
Similar articles
-
Neurosteroid withdrawal regulates GABA-A receptor α4-subunit expression and seizure susceptibility by activation of progesterone receptor-independent early growth response factor-3 pathway.Neuroscience. 2010 Oct 27;170(3):865-80. doi: 10.1016/j.neuroscience.2010.07.037. Epub 2010 Jul 27. Neuroscience. 2010. PMID: 20670676 Free PMC article.
-
Estrous cycle regulation of extrasynaptic δ-containing GABA(A) receptor-mediated tonic inhibition and limbic epileptogenesis.J Pharmacol Exp Ther. 2013 Jul;346(1):146-60. doi: 10.1124/jpet.113.203653. Epub 2013 May 10. J Pharmacol Exp Ther. 2013. PMID: 23667248 Free PMC article.
-
Neurosteroid synthesis-mediated regulation of GABA(A) receptors: relevance to the ovarian cycle and stress.J Neurosci. 2007 Feb 28;27(9):2155-62. doi: 10.1523/JNEUROSCI.4945-06.2007. J Neurosci. 2007. PMID: 17329412 Free PMC article.
-
Neurosteroid interactions with synaptic and extrasynaptic GABA(A) receptors: regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability.Psychopharmacology (Berl). 2013 Nov;230(2):151-88. doi: 10.1007/s00213-013-3276-5. Epub 2013 Sep 27. Psychopharmacology (Berl). 2013. PMID: 24071826 Free PMC article. Review.
-
Neurosteroids and their role in sex-specific epilepsies.Neurobiol Dis. 2014 Dec;72 Pt B:198-209. doi: 10.1016/j.nbd.2014.06.010. Epub 2014 Jun 21. Neurobiol Dis. 2014. PMID: 24960208 Free PMC article. Review.
Cited by
-
Anti-apoptotic Actions of Allopregnanolone and Ganaxolone Mediated Through Membrane Progesterone Receptors (PAQRs) in Neuronal Cells.Front Endocrinol (Lausanne). 2020 Jun 24;11:417. doi: 10.3389/fendo.2020.00417. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32670200 Free PMC article. Review.
-
3β-Methyl-Neurosteroid Analogs Are Preferential Positive Allosteric Modulators and Direct Activators of Extrasynaptic δ-Subunit γ-Aminobutyric Acid Type A Receptors in the Hippocampus Dentate Gyrus Subfield.J Pharmacol Exp Ther. 2018 Jun;365(3):583-601. doi: 10.1124/jpet.117.246660. Epub 2018 Mar 30. J Pharmacol Exp Ther. 2018. PMID: 29602830 Free PMC article.
-
Neurosteroid Metabolites of Gonadal Steroid Hormones in Neuroprotection: Implications for Sex Differences in Neurodegenerative Disease.Front Mol Neurosci. 2018 Oct 5;11:359. doi: 10.3389/fnmol.2018.00359. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30344476 Free PMC article. Review.
-
Neurosteroid Actions in Memory and Neurologic/Neuropsychiatric Disorders.Front Endocrinol (Lausanne). 2019 Apr 9;10:169. doi: 10.3389/fendo.2019.00169. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31024441 Free PMC article. Review.
-
Neurosteroid replacement therapy for catamenial epilepsy, postpartum depression and neuroendocrine disorders in women.J Neuroendocrinol. 2022 Feb;34(2):e13028. doi: 10.1111/jne.13028. Epub 2021 Sep 10. J Neuroendocrinol. 2022. PMID: 34506047 Free PMC article. Review.
References
-
- Backstrom T, Zetterlund B, Blom S, Romano M. Effects of intravenous progesterone infusions on the epileptic discharge frequency in women with partial epilepsy. Acta Neurol Scand. 1984;69:240–248. - PubMed
-
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABA-A receptors. Neuropharmacology. 2002;43:651–661. - PubMed
-
- Biggio G, Follesa P, Sanna E, Purdy RH, Concas A. GABA-A receptor plasticity during long-term exposure to and withdrawal from progesterone. Int Rev Neurobiol. 2001;46:207–241. - PubMed
-
- Bonuccelli U, Melis GB, Paoletti AM, Fioretti P, Murri L, Muratorio A. Unbalanced progesterone and estradiol secretion in catamenial epilepsy. Epilepsy Res. 1989;3:100–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

