An alternative sensor-based method for glucose monitoring in children and young people with diabetes
- PMID: 28137708
- PMCID: PMC5466923
- DOI: 10.1136/archdischild-2016-311530
An alternative sensor-based method for glucose monitoring in children and young people with diabetes
Abstract
Objective: To determine accuracy, safety and acceptability of the FreeStyle Libre Flash Glucose Monitoring System in the paediatric population.
Design, setting and patients: Eighty-nine study participants, aged 4-17 years, with type 1 diabetes were enrolled across 9 diabetes centres in the UK. A factory calibrated sensor was inserted on the back of the upper arm and used for up to 14 days. Sensor glucose measurements were compared with capillary blood glucose (BG) measurements. Sensor results were masked to participants.
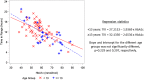
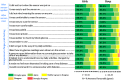
Results: Clinical accuracy of sensor results versus BG results was demonstrated, with 83.8% of results in zone A and 99.4% of results in zones A and B of the consensus error grid. Overall mean absolute relative difference (MARD) was 13.9%. Sensor accuracy was unaffected by patient factors such as age, body weight, sex, method of insulin administration or time of use (day vs night). Participants were in the target glucose range (3.9-10.0 mmol/L) ∼50% of the time (mean 12.1 hours/day), with an average of 2.2 hours/day and 9.5 hours/day in hypoglycaemia and hyperglycaemia, respectively. Sensor application, wear/use of the device and comparison to self-monitoring of blood glucose were rated favourably by most participants/caregivers (84.3-100%). Five device related adverse events were reported across a range of participant ages.
Conclusions: Accuracy, safety and user acceptability of the FreeStyle Libre System were demonstrated for the paediatric population. Accuracy of the system was unaffected by subject characteristics, making it suitable for a broad range of children and young people with diabetes.
Trial registration number: NCT02388815.
Keywords: Diabetes; General Paediatrics; Monitoring; Technology.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Competing interests: All authors' institutions received financial support from Abbott Diabetes Care to conduct the clinical trial. TR received honoraria/fees for consulting from Abbott Diabetes Care outside of the submitted work. The study sponsor (Abbott Diabetes Care) designed the study protocol in collaboration with the principal investigator and provided all study materials. The sponsor was involved in collecting data and reporting results. The sponsor also gave approval to submit for publication. The corresponding author had full access to all the data in the study and, together with all authors, had final responsibility for the decision to submit for publication.
Figures

References
-
- Diabetes Control and Complication Trial Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin dependent diabetes mellitus: diabetes control and complications trial. J Pediatr 1994;125:177–88. 10.1016/S0022-3476(94)70190-3 - DOI - PubMed
-
- International Diabetes Federation. Global IDF/ISPAD guideline for diabetes in childhood and adolescence, 2011.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
