Structure and Function of the PiuA and PirA Siderophore-Drug Receptors from Pseudomonas aeruginosa and Acinetobacter baumannii
- PMID: 28137795
- PMCID: PMC5365723
- DOI: 10.1128/AAC.02531-16
Structure and Function of the PiuA and PirA Siderophore-Drug Receptors from Pseudomonas aeruginosa and Acinetobacter baumannii
Abstract
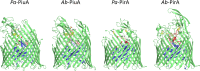
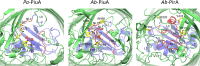
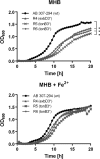
The outer membrane of Gram-negative bacteria presents an efficient barrier to the permeation of antimicrobial molecules. One strategy pursued to circumvent this obstacle is to hijack transport systems for essential nutrients, such as iron. BAL30072 and MC-1 are two monobactams conjugated to a dihydroxypyridone siderophore that are active against Pseudomonas aeruginosa and Acinetobacter baumannii Here, we investigated the mechanism of action of these molecules in A. baumannii We identified two novel TonB-dependent receptors, termed Ab-PiuA and Ab-PirA, that are required for the antimicrobial activity of both agents. Deletion of either piuA or pirA in A. baumannii resulted in 4- to 8-fold-decreased susceptibility, while their overexpression in the heterologous host P. aeruginosa increased susceptibility to the two siderophore-drug conjugates by 4- to 32-fold. The crystal structures of PiuA and PirA from A. baumannii and their orthologues from P. aeruginosa were determined. The structures revealed similar architectures; however, structural differences between PirA and PiuA point to potential differences between their cognate siderophore ligands. Spontaneous mutants, selected upon exposure to BAL30072, harbored frameshift mutations in either the ExbD3 or the TonB3 protein of A. baumannii, forming the cytoplasmic-membrane complex providing the energy for the siderophore translocation process. The results of this study provide insight for the rational design of novel siderophore-drug conjugates against problematic Gram-negative pathogens.
Keywords: Acinetobacter baumannii; Pseudomonas aeruginosa; siderophore-drug receptor.
Copyright © 2017 American Society for Microbiology.
Figures


Similar articles
-
TonB-Dependent Receptor Repertoire of Pseudomonas aeruginosa for Uptake of Siderophore-Drug Conjugates.Antimicrob Agents Chemother. 2018 May 25;62(6):e00097-18. doi: 10.1128/AAC.00097-18. Print 2018 Jun. Antimicrob Agents Chemother. 2018. PMID: 29555629 Free PMC article.
-
Involvement of Fe uptake systems and AmpC β-lactamase in susceptibility to the siderophore monosulfactam BAL30072 in Pseudomonas aeruginosa.Antimicrob Agents Chemother. 2013 May;57(5):2095-102. doi: 10.1128/AAC.02474-12. Epub 2013 Feb 19. Antimicrob Agents Chemother. 2013. PMID: 23422914 Free PMC article.
-
In vitro activity of the siderophore monosulfactam BAL30072 against contemporary Gram-negative pathogens from New York City, including multidrug-resistant isolates.Int J Antimicrob Agents. 2014 Jun;43(6):527-32. doi: 10.1016/j.ijantimicag.2014.02.017. Epub 2014 Apr 13. Int J Antimicrob Agents. 2014. PMID: 24796217
-
Multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii: resistance mechanisms and implications for therapy.Expert Rev Anti Infect Ther. 2010 Jan;8(1):71-93. doi: 10.1586/eri.09.108. Expert Rev Anti Infect Ther. 2010. PMID: 20014903 Review.
-
[Interpretive reading of the non-fermenting gram-negative bacilli antibiogram].Enferm Infecc Microbiol Clin. 2010 Dec;28(10):726-36. doi: 10.1016/j.eimc.2010.05.001. Epub 2010 Jun 26. Enferm Infecc Microbiol Clin. 2010. PMID: 20579775 Review. Spanish.
Cited by
-
The Esterase PfeE, the Achilles' Heel in the Battle for Iron between Pseudomonas aeruginosa and Escherichia coli.Int J Mol Sci. 2021 Mar 10;22(6):2814. doi: 10.3390/ijms22062814. Int J Mol Sci. 2021. PMID: 33802163 Free PMC article.
-
Promysalin Elicits Species-Selective Inhibition of Pseudomonas aeruginosa by Targeting Succinate Dehydrogenase.J Am Chem Soc. 2018 Feb 7;140(5):1774-1782. doi: 10.1021/jacs.7b11212. Epub 2018 Jan 24. J Am Chem Soc. 2018. PMID: 29300464 Free PMC article.
-
Next-generation strategy for treating drug resistant bacteria: Antibiotic hybrids.Indian J Med Res. 2019 Feb;149(2):97-106. doi: 10.4103/ijmr.IJMR_755_18. Indian J Med Res. 2019. PMID: 31219074 Free PMC article. Review.
-
Genomics of Acinetobacter baumannii iron uptake.Microb Genom. 2023 Aug;9(8):mgen001080. doi: 10.1099/mgen.0.001080. Microb Genom. 2023. PMID: 37549061 Free PMC article.
-
Acinetobacter baumannii can use multiple siderophores for iron acquisition, but only acinetobactin is required for virulence.PLoS Pathog. 2020 Oct 19;16(10):e1008995. doi: 10.1371/journal.ppat.1008995. eCollection 2020 Oct. PLoS Pathog. 2020. PMID: 33075115 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

