Time Trends in Sputum Mycobacterial Load and Two-Day Bactericidal Activity of Isoniazid-Containing Antituberculosis Therapies
- PMID: 28137798
- PMCID: PMC5365658
- DOI: 10.1128/AAC.02088-16
Time Trends in Sputum Mycobacterial Load and Two-Day Bactericidal Activity of Isoniazid-Containing Antituberculosis Therapies
Abstract
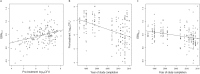
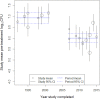
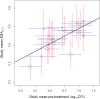
Recent early bactericidal activity (EBA) studies of isoniazid-based antituberculosis therapies have shown a lower EBA over the first two treatment days than in earlier years. To quantify this trend and evaluate factors contributing to it, we extracted individual data from 18 studies with a total of 182 participants using isoniazid-containing therapies between 1992 and 2015 at a single site and laboratory in Cape Town, South Africa. We recalculated EBA as the daily fall in CFU per milliliter sputum up to day 2 of therapy (EBA0-2) for individual patients and treatment groups and used mixed-effects linear models to investigate the correlation between pretreatment CFU, EBA0-2, and year of study. We found that mean pretreatment CFU and year of study accounted for 46% and 47%, respectively, of the variation in mean EBA0-2 Mean pretreatment CFU differed between the periods 1992 to 2001 and 2007 to 2015 by 0.92 log10 CFU (95% confidence interval [CI], 0.57 to 1.28; P < 0.0001). On average, pretreatment CFU dropped by 0.053 log10 CFU (95% CI, 0.029 to 0.076; P = 0.0004) and EBA0-2 by 0.012 log10 CFU (95% CI, 0.006 to 0.018; P = 0.001) per year. The EBA0-2 of isoniazid-based antituberculosis therapy is strongly correlated with baseline mycobacterial load and shows a declining trend over the past 2 decades.
Keywords: early bactericidal activity; isoniazid; tuberculosis.
Copyright © 2017 American Society for Microbiology.
Figures
Similar articles
-
Early bactericidal activity of ethambutol, pyrazinamide and the fixed combination of isoniazid, rifampicin and pyrazinamide (Rifater) in patients with pulmonary tuberculosis.S Afr Med J. 1996 Feb;86(2):155-8. S Afr Med J. 1996. PMID: 8619142
-
Early Bactericidal Activity of AZD5847 in Patients with Pulmonary Tuberculosis.Antimicrob Agents Chemother. 2016 Oct 21;60(11):6591-6599. doi: 10.1128/AAC.01163-16. Print 2016 Nov. Antimicrob Agents Chemother. 2016. PMID: 27550361 Free PMC article. Clinical Trial.
-
Early bactericidal activity of a moxifloxacin and isoniazid combination in smear-positive pulmonary tuberculosis.J Antimicrob Chemother. 2005 Dec;56(6):1169-71. doi: 10.1093/jac/dki376. Epub 2005 Oct 13. J Antimicrob Chemother. 2005. PMID: 16223939
-
Early bactericidal activity of antituberculosis agents.Expert Rev Anti Infect Ther. 2003 Jun;1(1):141-55. doi: 10.1586/14787210.1.1.141. Expert Rev Anti Infect Ther. 2003. PMID: 15482107 Review.
-
The early bactericidal activity of anti-tuberculosis drugs: a literature review.Tuberculosis (Edinb). 2008 Aug;88 Suppl 1:S75-83. doi: 10.1016/S1472-9792(08)70038-6. Tuberculosis (Edinb). 2008. PMID: 18762155 Review.
Cited by
-
Efficacy and Safety of High-Dose Rifampin in Pulmonary Tuberculosis. A Randomized Controlled Trial.Am J Respir Crit Care Med. 2018 Sep 1;198(5):657-666. doi: 10.1164/rccm.201712-2524OC. Am J Respir Crit Care Med. 2018. PMID: 29954183 Free PMC article. Clinical Trial.
-
High-dose isoniazid for TB with low-to-moderate isoniazid resistance after 1 week of treatment.JAC Antimicrob Resist. 2025 May 22;7(3):dlaf072. doi: 10.1093/jacamr/dlaf072. eCollection 2025 Jun. JAC Antimicrob Resist. 2025. PMID: 40406736 Free PMC article.
-
High-Dose Isoniazid Lacks EARLY Bactericidal Activity against Isoniazid-resistant Tuberculosis Mediated by katG Mutations: A Randomized Phase II Clinical Trial.Am J Respir Crit Care Med. 2024 Aug 1;210(3):343-351. doi: 10.1164/rccm.202311-2004OC. Am J Respir Crit Care Med. 2024. PMID: 38564365 Free PMC article. Clinical Trial.
-
Assessing whether isoniazid is essential during the first 14 days of tuberculosis therapy: a phase 2a, open-label, randomised controlled trial.Lancet Microbe. 2020 Jun;1(2):e84-e92. doi: 10.1016/s2666-5247(20)30011-2. Epub 2020 Jun 8. Lancet Microbe. 2020. PMID: 33834177 Free PMC article. Clinical Trial.
-
Fourteen-Day Bactericidal Activity, Safety, and Pharmacokinetics of Linezolid in Adults with Drug-Sensitive Pulmonary Tuberculosis.Antimicrob Agents Chemother. 2020 Mar 24;64(4):e02012-19. doi: 10.1128/AAC.02012-19. Print 2020 Mar 24. Antimicrob Agents Chemother. 2020. PMID: 31988102 Free PMC article. Clinical Trial.
References
-
- Jindani A, Aber VR, Edwards EA, Mitchison DA. 1980. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am Rev Respir Dis 121:939–949. - PubMed
-
- Sirgel FA, Botha FJ, Parkin DP, Van De Wal BW, Donald PR, Clark PK, Mitchison DA. 1993. The early bactericidal activity of rifabutin in patients with pulmonary tuberculosis measured by sputum viable counts: a new method of drug assessment. J Antimicrob Chemother 32:867–875. doi:10.1093/jac/32.6.867. - DOI - PubMed
-
- Donald PR, Sirgel FA, Botha FJ, Seifart HI, Parkin DP, Vandenplas ML, Van de Wal BW, Maritz JS, Mitchison DA. 1997. The early bactericidal activity of isoniazid related to its dose size in pulmonary tuberculosis. Am J Respir Crit Care Med 156:895–900. doi:10.1164/ajrccm.156.3.9609132. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

