Some Synonymous and Nonsynonymous gyrA Mutations in Mycobacterium tuberculosis Lead to Systematic False-Positive Fluoroquinolone Resistance Results with the Hain GenoType MTBDR sl Assays
- PMID: 28137812
- PMCID: PMC5365657
- DOI: 10.1128/AAC.02169-16
Some Synonymous and Nonsynonymous gyrA Mutations in Mycobacterium tuberculosis Lead to Systematic False-Positive Fluoroquinolone Resistance Results with the Hain GenoType MTBDR sl Assays
Abstract
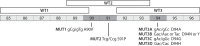
In this study, using the Hain GenoType MTBDRsl assays (versions 1 and 2), we found that some nonsynonymous and synonymous mutations in gyrA in Mycobacterium tuberculosis result in systematic false-resistance results to fluoroquinolones by preventing the binding of wild-type probes. Moreover, such mutations can prevent the binding of mutant probes designed for the identification of specific resistance mutations. Although these mutations are likely rare globally, they occur in approximately 7% of multidrug-resistant tuberculosis strains in some settings.
Keywords: Hain GenoType MTBDRsl; Mycobacterium tuberculosis; fluoroquinolones.
Copyright © 2017 Ajileye et al.
Figures

Similar articles
-
Pseudo-outbreak of pre-extensively drug-resistant (Pre-XDR) tuberculosis in Kinshasa: collateral damage caused by false detection of fluoroquinolone resistance by GenoType MTBDRsl.J Clin Microbiol. 2014 Aug;52(8):2876-80. doi: 10.1128/JCM.00398-14. Epub 2014 May 28. J Clin Microbiol. 2014. PMID: 24871222 Free PMC article.
-
Fluoroquinolone resistance and mutational profile of gyrA in pulmonary MDR tuberculosis patients.BMC Pulm Med. 2020 May 11;20(1):138. doi: 10.1186/s12890-020-1172-4. BMC Pulm Med. 2020. PMID: 32393213 Free PMC article.
-
Emergence of Specific gyrA Mutations Associated High-Level Fluoroquinolone-Resistant Mycobacterium tuberculosis among Multidrug-Resistant Tuberculosis Cases in North India.Microb Drug Resist. 2021 May;27(5):647-651. doi: 10.1089/mdr.2020.0240. Epub 2020 Sep 29. Microb Drug Resist. 2021. PMID: 32991238
-
A systematic review of gyrase mutations associated with fluoroquinolone-resistant Mycobacterium tuberculosis and a proposed gyrase numbering system.J Antimicrob Chemother. 2012 Apr;67(4):819-31. doi: 10.1093/jac/dkr566. Epub 2012 Jan 25. J Antimicrob Chemother. 2012. PMID: 22279180 Free PMC article.
-
Mechanisms of fluoroquinolone resistance in Mycobacterium tuberculosis.Yi Chuan. 2016 Oct 20;38(10):918-927. doi: 10.16288/j.yczz.16-136. Yi Chuan. 2016. PMID: 27806933 Review.
Cited by
-
Detection of Second Line Drug Resistance among Drug Resistant Mycobacterium Tuberculosis Isolates in Botswana.Pathogens. 2019 Oct 28;8(4):208. doi: 10.3390/pathogens8040208. Pathogens. 2019. PMID: 31661825 Free PMC article.
-
In silico evaluation of WHO-endorsed molecular methods to detect drug resistant tuberculosis.Sci Rep. 2022 Oct 22;12(1):17741. doi: 10.1038/s41598-022-21025-6. Sci Rep. 2022. PMID: 36273016 Free PMC article.
-
Prediction of drug resistance by Sanger sequencing of Mycobacterium tuberculosis complex strains isolated from multidrug resistant tuberculosis suspect patients in Ethiopia.PLoS One. 2022 Aug 5;17(8):e0271508. doi: 10.1371/journal.pone.0271508. eCollection 2022. PLoS One. 2022. PMID: 35930613 Free PMC article.
-
Direct detection of drug-resistant Mycobacterium tuberculosis using targeted next generation sequencing.Front Public Health. 2023 Jun 29;11:1206056. doi: 10.3389/fpubh.2023.1206056. eCollection 2023. Front Public Health. 2023. PMID: 37457262 Free PMC article.
-
Predicting antibiotic resistance in complex protein targets using alchemical free energy methods.J Comput Chem. 2022 Oct 5;43(26):1771-1782. doi: 10.1002/jcc.26979. Epub 2022 Aug 25. J Comput Chem. 2022. PMID: 36054249 Free PMC article.
References
-
- Tagliani E, Cabibbe AM, Miotto P, Borroni E, Toro JC, Mansjo M, Hoffner S, Hillemann D, Zalutskaya A, Skrahina A, Cirillo DM. 2015. Diagnostic performance of the new version of GenoType MTBDRsl (V2.0) assay for detection of resistance to fluoroquinolones and second line injectable drugs: a multicenter study. J Clin Microbiol 53:2961–2969. doi:10.1128/JCM.01257-15. - DOI - PMC - PubMed
-
- World Health Organization. 2016. The use of molecular line probe assays for the detection of resistance to second-line anti-tuberculosis drugs. Policy guidance. http://www.who.int/tb/areas-of-work/laboratory/WHOPolicyStatementSLLPA.p... Accessed 31 July 2016.
-
- World Health Organization. 2016. Online annexes (5–8) to WHO policy guidance: the use of molecular line probe assay for the detection of resistance to second-line anti-tuberculosis drugs. http://www.who.int/tb/areas-of-work/laboratory/OnlineAnnexes_MTBDRsl.pdf.... Accessed 2 August 2016.
-
- World Health Organization. 2016. WHO treatment guidelines for drug-resistant tuberculosis, 2016 update. World Health Organization, Geneva, Switzerland: https://www.ncbi.nlm.nih.gov/books/NBK390455/ Accessed 3 March 2016.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

