DSCAM promotes axon fasciculation and growth in the developing optic pathway
- PMID: 28137836
- PMCID: PMC5321013
- DOI: 10.1073/pnas.1618606114
DSCAM promotes axon fasciculation and growth in the developing optic pathway
Abstract
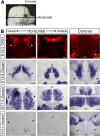
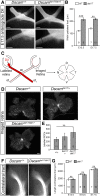
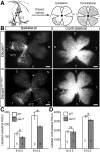
Although many aspects of optic pathway development are beginning to be understood, the mechanisms promoting the growth of retinal ganglion cell (RGC) axons toward visual targets remain largely unknown. Down syndrome cell adhesion molecule (Dscam) is expressed by mouse RGCs shortly after they differentiate at embryonic day 12 and is essential for multiple aspects of postnatal visual system development. Here we show that Dscam is also required during embryonic development for the fasciculation and growth of RGC axons. Dscam is expressed along the developing optic pathway in a pattern consistent with a role in regulating RGC axon outgrowth. In mice carrying spontaneous mutations in Dscam (Dscamdel17 ; Dscam2J), RGC axons pathfind normally, but growth from the chiasm toward their targets is impaired, resulting in a delay in RGC axons reaching the dorsal thalamus compared with that seen in wild-type littermates. Conversely, Dscam gain of function results in exuberant growth into the dorsal thalamus. The growth of ipsilaterally projecting axons is particularly affected. Axon organization in the optic chiasm and tract and RGC growth cone morphologies are also altered in Dscam mutants. In vitro DSCAM promotes RGC axon growth and fasciculation, and can act independently of cell contact. In vitro and in situ DSCAM is required both in the RGC axons and in their environment for the promotion of axon outgrowth, consistent with a homotypic mode of action. These findings identify DSCAM as a permissive signal that promotes the growth and fasciculation of RGC axons, controlling the timing of when RGC axons reach their targets.
Keywords: axon guidance; development; growth cone; optic chiasm; visual system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












References
-
- Petrovic M, Schmucker D. Axonal wiring in neural development: Target-independent mechanisms help to establish precision and complexity. BioEssays. 2015;37(9):996–1004. - PubMed
-
- Schmucker D, Chen B. Dscam and DSCAM: Complex genes in simple animals, complex animals yet simple genes. Genes Dev. 2009;23(2):147–156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

