Alterations of p11 in brain tissue and peripheral blood leukocytes in Parkinson's disease
- PMID: 28137881
- PMCID: PMC5347629
- DOI: 10.1073/pnas.1621218114
Alterations of p11 in brain tissue and peripheral blood leukocytes in Parkinson's disease
Abstract
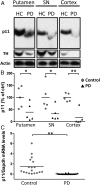
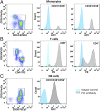
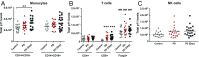
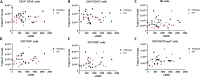
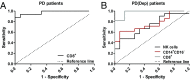
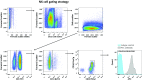
Individuals with Parkinson's disease (PD) often suffer from comorbid depression. P11 (S100A10), a member of the S100 family of proteins, is expressed widely throughout the body and is involved in major depressive disorder and antidepressant response. Central p11 levels are reduced in postmortem tissue from depressed individuals; however, p11 has not yet been investigated in PD patients with depression or those without depression. We investigated p11 levels in postmortem PD brains and assessed whether peripheral p11 levels correlate with disease severity. Substantia nigra, putamen, and cortical p11 protein levels were assessed in postmortem brain samples from PD patients and matched controls. In a different set of postmortem brains, p11 mRNA expression was measured in dopaminergic cells from the substantia nigra. Both p11 protein and mRNA levels were decreased in PD patients. Peripheral p11 protein levels were investigated in distinct leukocyte populations from PD patients with depression and those without depression. Monocyte, natural killer (NK) cell, and cytotoxic T-cell p11 levels were positively associated with the severity of PD, and NK cell p11 levels were positively associated with depression scores. Given that inflammation plays a role in both PD and depression, it is intriguing that peripheral p11 levels are altered in immune cells in both conditions. Our data provide insight into the pathological alterations occurring centrally and peripherally in PD. Moreover, if replicated in other cohorts, p11 could be an easily accessible biomarker for monitoring the severity of PD, especially in the context of comorbid depression.
Keywords: Parkinson's disease; S100A10; annexin II light chain; biomarker; depression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


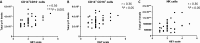


Comment in
-
The depressed brain in Parkinson's disease: Implications for an inflammatory biomarker.Proc Natl Acad Sci U S A. 2017 Mar 21;114(12):3004-3005. doi: 10.1073/pnas.1700737114. Epub 2017 Mar 10. Proc Natl Acad Sci U S A. 2017. PMID: 28283656 Free PMC article. No abstract available.
References
-
- Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386(9996):896–912. - PubMed
-
- Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson’s disease: Dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8(5):464–474. - PubMed
-
- Aarsland D, Påhlhagen S, Ballard CG, Ehrt U, Svenningsson P. Depression in Parkinson disease: Epidemiology, mechanisms and management. Nat Rev Neurol. 2011;8(1):35–47. - PubMed
-
- Brooks DJ, Pavese N. Imaging biomarkers in Parkinson’s disease. Prog Neurobiol. 2011;95(4):614–628. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

