cGAS is essential for the antitumor effect of immune checkpoint blockade
- PMID: 28137885
- PMCID: PMC5320994
- DOI: 10.1073/pnas.1621363114
cGAS is essential for the antitumor effect of immune checkpoint blockade
Abstract
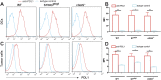
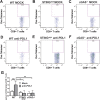
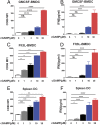
cGMP-AMP (cGAMP) synthase (cGAS) is a cytosolic DNA sensor that activates innate immune responses. cGAS catalyzes the synthesis of cGAMP, which functions as a second messenger that binds and activates the adaptor protein STING to induce type I interferons (IFNs) and other immune modulatory molecules. Here we show that cGAS is indispensable for the antitumor effect of immune checkpoint blockade in mice. Wild-type, but not cGAS-deficient, mice exhibited slower growth of B16 melanomas in response to a PD-L1 antibody treatment. Consistently, intramuscular delivery of cGAMP inhibited melanoma growth and prolonged the survival of the tumor-bearing mice. The combination of cGAMP and PD-L1 antibody exerted stronger antitumor effects than did either treatment alone. cGAMP treatment activated dendritic cells and enhanced cross-presentation of tumor-associated antigens to CD8 T cells. These results indicate that activation of the cGAS pathway is important for intrinsic antitumor immunity and that cGAMP may be used directly for cancer immunotherapy.
Keywords: PD-L1; STING; cGAMP; cGAS; cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

