Loss-of-function mutations in the ATP13A2/PARK9 gene cause complicated hereditary spastic paraplegia (SPG78)
- PMID: 28137957
- PMCID: PMC5278306
- DOI: 10.1093/brain/aww307
Loss-of-function mutations in the ATP13A2/PARK9 gene cause complicated hereditary spastic paraplegia (SPG78)
Abstract
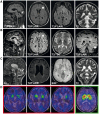
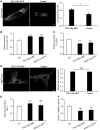
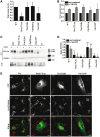
Hereditary spastic paraplegias are heterogeneous neurodegenerative disorders characterized by progressive spasticity of the lower limbs due to degeneration of the corticospinal motor neurons. In a Bulgarian family with three siblings affected by complicated hereditary spastic paraplegia, we performed whole exome sequencing and homozygosity mapping and identified a homozygous p.Thr512Ile (c.1535C > T) mutation in ATP13A2. Molecular defects in this gene have been causally associated with Kufor-Rakeb syndrome (#606693), an autosomal recessive form of juvenile-onset parkinsonism, and neuronal ceroid lipofuscinosis (#606693), a neurodegenerative disorder characterized by the intracellular accumulation of autofluorescent lipopigments. Further analysis of 795 index cases with hereditary spastic paraplegia and related disorders revealed two additional families carrying truncating biallelic mutations in ATP13A2. ATP13A2 is a lysosomal P5-type transport ATPase, the activity of which critically depends on catalytic autophosphorylation. Our biochemical and immunocytochemical experiments in COS-1 and HeLa cells and patient-derived fibroblasts demonstrated that the hereditary spastic paraplegia-associated mutations, similarly to the ones causing Kufor-Rakeb syndrome and neuronal ceroid lipofuscinosis, cause loss of ATP13A2 function due to transcript or protein instability and abnormal intracellular localization of the mutant proteins, ultimately impairing the lysosomal and mitochondrial function. Moreover, we provide the first biochemical evidence that disease-causing mutations can affect the catalytic autophosphorylation activity of ATP13A2. Our study adds complicated hereditary spastic paraplegia (SPG78) to the clinical continuum of ATP13A2-associated neurological disorders, which are commonly hallmarked by lysosomal and mitochondrial dysfunction. The disease presentation in our patients with hereditary spastic paraplegia was dominated by an adult-onset lower-limb predominant spastic paraparesis. Cognitive impairment was present in most of the cases and ranged from very mild deficits to advanced dementia with fronto-temporal characteristics. Nerve conduction studies revealed involvement of the peripheral motor and sensory nerves. Only one of five patients with hereditary spastic paraplegia showed clinical indication of extrapyramidal involvement in the form of subtle bradykinesia and slight resting tremor. Neuroimaging cranial investigations revealed pronounced vermian and hemispheric cerebellar atrophy. Notably, reduced striatal dopamine was apparent in the brain of one of the patients, who had no clinical signs or symptoms of extrapyramidal involvement.
Keywords: Kufor-Rakeb syndrome; hereditary spastic paraplegia (HSP); lysosomes; neuronal ceroid lipofuscinosis; parkinsonism.
© The Author (2016). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


Comment in
-
Complicated hereditary spastic paraplegia due to ATP13A2 mutations: what's in a name?Brain. 2017 Dec 1;140(12):e73. doi: 10.1093/brain/awx280. Brain. 2017. PMID: 29112699 No abstract available.
-
Reply: Complicated hereditary spastic paraplegia due to ATP13A2 mutations: what's in a name?Brain. 2017 Dec 1;140(12):e74. doi: 10.1093/brain/awx282. Brain. 2017. PMID: 29112700 Free PMC article. No abstract available.
References
-
- Anheim M, Lagier-Tourenne C, Stevanin G, Fleury M, Durr A, Namer IJ, et al.SPG11 spastic paraplegia: a new cause of juvenile parkinsonism. J Neurol 2009; 256: 104–8. - PubMed
-
- Arif B, Kumar KR, Seibler P, Vulinovic F, Fatima A, Winkler S, et al.A novel OPA3 mutation revealed by exome sequencing: an example of reverse phenotyping. JAMA Neurol 2013; 70: 783–7. - PubMed
-
- Behrens MI, Bruggemann N, Chana P, Venegas P, Kagi M, Parrao T, et al.Clinical spectrum of Kufor-Rakeb syndrome in the Chilean kindred with ATP13A2 mutations. Mov Disord 2010; 25: 1929–37. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

