Selenomethionine Mitigates Cognitive Decline by Targeting Both Tau Hyperphosphorylation and Autophagic Clearance in an Alzheimer's Disease Mouse Model
- PMID: 28137967
- PMCID: PMC6596838
- DOI: 10.1523/JNEUROSCI.3229-16.2017
Selenomethionine Mitigates Cognitive Decline by Targeting Both Tau Hyperphosphorylation and Autophagic Clearance in an Alzheimer's Disease Mouse Model
Abstract
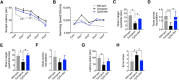
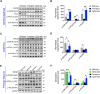
Tau pathology was recently identified as a key driver of disease progression and an attractive therapeutic target in Alzheimer's disease (AD). Selenomethionine (Se-Met), a major bioactive form of selenium (Se) in organisms with significant antioxidant capacity, reduced the levels of total tau and hyperphosphorylated tau and ameliorated cognitive deficits in younger triple transgenic AD (3xTg-AD) mice. Whether Se-Met has a similar effect on tau pathology and the specific mechanism of action in older 3xTg-AD mice remains unknown. Autophagy is a major self-degradative process to maintain cellular homeostasis and function. Autophagic dysfunction has been implicated in the pathogenesis of multiple age-dependent diseases, including AD. Modulation of autophagy has been shown to retard the accumulation of misfolded and aggregated proteins and to delay the progression of AD. Here, we found that 3xTg-AD mice showed significant improvement in cognitive ability after a 3-month treatment with Se-Met beginning at 8 months of age. In addition to attenuating the hyperphosphorylation of tau by modulating the activity of Akt/glycogen synthase kinase-3β and protein phosphatase 2A, Se-Met-induced reduction of tau was also mediated by an autophagy-based pathway. Specifically, Se-Met improved the initiation of autophagy via the AMP-activated protein kinase-mTOR (mammalian target of rapamycin) signaling pathway and enhanced autophagic flux to promote the clearance of tau in 3xTg-AD mice and primary 3xTg neurons. Thus, our results demonstrate for the first time that Se-Met mitigates cognitive decline by targeting both the hyperphosphorylation of tau and the autophagic clearance of tau in AD mice. These data strongly support Se-Met as a potent nutraceutical for AD therapy.SIGNIFICANCE STATEMENT Selenium has been widely recognized as a vital trace element abundant in the brain with effects of antioxidant, anticancer, and anti-inflammation. In this study, we report that selenomethionine rescues spatial learning and memory impairments in aged 3xTg-AD mice via decreasing the level of tau protein and tau hyperphosphorylation. We find that selenomethionine promotes the initiation of autophagy via the AMPK-mTOR pathway and enhances autophagic flux, thereby facilitating tau clearance in vivo and in vitro We have now identified an additional, novel mechanism by which selenomethionine improves the cognitive function of AD mice. Specifically, our data suggest the effect of selenium/selenomethionine on an autophagic pathway in Alzheimer's disease.
Keywords: Alzheimer's disease; autophagy; hyperphosphorylated tau; selenomethionine; tau; tauopathy.
Copyright © 2017 the authors 0270-6474/17/372449-14$15.00/0.
Figures






References
-
- Avrahami L, Farfara D, Shaham-Kol M, Vassar R, Frenkel D, Eldar-Finkelman H (2013) Inhibition of glycogen synthase kinase-3 ameliorates beta-amyloid pathology and restores lysosomal acidification and mammalian target of rapamycin activity in the Alzheimer disease mouse model: in vivo and in vitro studies. J Biol Chem 288:1295–1306. 10.1074/jbc.M112.409250 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
