Transcranial Alternating Current Stimulation Attenuates Neuronal Adaptation
- PMID: 28137971
- PMCID: PMC5354346
- DOI: 10.1523/JNEUROSCI.2266-16.2016
Transcranial Alternating Current Stimulation Attenuates Neuronal Adaptation
Abstract
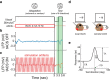
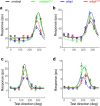
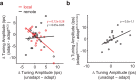
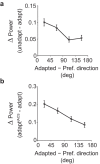
We previously showed that brief application of 2 mA (peak-to-peak) transcranial currents alternating at 10 Hz significantly reduces motion adaptation in humans. This is but one of many behavioral studies showing that weak currents applied to the scalp modulate neural processing. Transcranial stimulation has been shown to improve perception, learning, and a range of clinical symptoms. Few studies, however, have measured the neural consequences of transcranial current stimulation. We capitalized on the strong link between motion perception and neural activity in the middle temporal (MT) area of the macaque monkey to study the neural mechanisms that underlie the behavioral consequences of transcranial alternating current stimulation. First, we observed that 2 mA currents generated substantial intracranial fields, which were much stronger in the stimulated hemisphere (0.12 V/m) than on the opposite side of the brain (0.03 V/m). Second, we found that brief application of transcranial alternating current stimulation at 10 Hz reduced spike-frequency adaptation of MT neurons and led to a broadband increase in the power spectrum of local field potentials. Together, these findings provide a direct demonstration that weak electric fields applied to the scalp significantly affect neural processing in the primate brain and that this includes a hitherto unknown mechanism that attenuates sensory adaptation.SIGNIFICANCE STATEMENT Transcranial stimulation has been claimed to improve perception, learning, and a range of clinical symptoms. Little is known, however, how transcranial current stimulation generates such effects, and the search for better stimulation protocols proceeds largely by trial and error. We investigated, for the first time, the neural consequences of stimulation in the monkey brain. We found that even brief application of alternating current stimulation reduced the effects of adaptation on single-neuron firing rates and local field potentials; this mechanistic insight explains previous behavioral findings and suggests a novel way to modulate neural information processing using transcranial currents. In addition, by developing an animal model to help understand transcranial stimulation, this study will aid the rational design of stimulation protocols for the treatment of mental illnesses, and the improvement of perception and learning.
Keywords: entrainment; local field potential; motion adaptation; motion after effect; neural mechanisms; transcranial alternating current stimulation.
Copyright © 2017 the authors 0270-6474/17/372325-11$15.00/0.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
