An Ultrastructural Study of the Thalamic Input to Layer 4 of Primary Motor and Primary Somatosensory Cortex in the Mouse
- PMID: 28137974
- PMCID: PMC6596845
- DOI: 10.1523/JNEUROSCI.2557-16.2017
An Ultrastructural Study of the Thalamic Input to Layer 4 of Primary Motor and Primary Somatosensory Cortex in the Mouse
Abstract
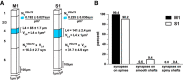
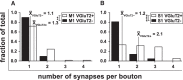
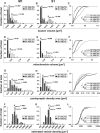
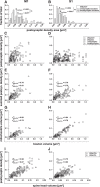
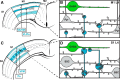
The traditional classification of primary motor cortex (M1) as an agranular area has been challenged recently when a functional layer 4 (L4) was reported in M1. L4 is the principal target for thalamic input in sensory areas, which raises the question of how thalamocortical synapses formed in M1 in the mouse compare with those in neighboring sensory cortex (S1). We identified thalamic boutons by their immunoreactivity for the vesicular glutamate transporter 2 (VGluT2) and performed unbiased disector counts from electron micrographs. We discovered that the thalamus contributed proportionately only half as many synapses to the local circuitry of L4 in M1 compared with S1. Furthermore, thalamic boutons in M1 targeted spiny dendrites exclusively, whereas ∼9% of synapses were formed with dendrites of smooth neurons in S1. VGluT2+ boutons in M1 were smaller and formed fewer synapses per bouton on average (1.3 vs 2.1) than those in S1, but VGluT2+ synapses in M1 were larger than in S1 (median postsynaptic density areas of 0.064 μm2 vs 0.042 μm2). In M1 and S1, thalamic synapses formed only a small fraction (12.1% and 17.2%, respectively) of all of the asymmetric synapses in L4. The functional role of the thalamic input to L4 in M1 has largely been neglected, but our data suggest that, as in S1, the thalamic input is amplified by the recurrent excitatory connections of the L4 circuits. The lack of direct thalamic input to inhibitory neurons in M1 may indicate temporal differences in the inhibitory gating in L4 of M1 versus S1.SIGNIFICANCE STATEMENT Classical interpretations of the function of primary motor cortex (M1) emphasize its lack of the granular layer 4 (L4) typical of sensory cortices. However, we show here that, like sensory cortex (S1), mouse M1 also has the canonical circuit motif of a core thalamic input to the middle cortical layer and that thalamocortical synapses form a small fraction (M1: 12%; S1: 17%) of all asymmetric synapses in L4 of both areas. Amplification of thalamic input by recurrent local circuits is thus likely to be a significant mechanism in both areas. Unlike M1, where thalamocortical boutons typically form a single synapse, thalamocortical boutons in S1 usually formed multiple synapses, which means they can be identified with high probability in the electron microscope without specific labeling.
Keywords: VGluT2; barrel cortex; electron microscopy; layer 4; motor cortex; thalamocortical.
Copyright © 2017 the authors 0270-6474/17/372435-14$15.00/0.
Figures




References
-
- Ahmed B, Anderson JC, Martin KA, Nelson JC (1997) Map of the synapses onto layer 4 basket cells of the primary visual cortex of the cat. J Comp Neurol 380:230–242. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
