Combining Chk1/2 Inhibition with Cetuximab and Radiation Enhances In Vitro and In Vivo Cytotoxicity in Head and Neck Squamous Cell Carcinoma
- PMID: 28138028
- PMCID: PMC5560482
- DOI: 10.1158/1535-7163.MCT-16-0352
Combining Chk1/2 Inhibition with Cetuximab and Radiation Enhances In Vitro and In Vivo Cytotoxicity in Head and Neck Squamous Cell Carcinoma
Abstract
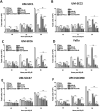
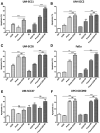
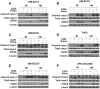
EGFR inhibition and radiotherapy are potent inducers of DNA damage. Checkpoint kinases 1 and 2 (Chk1/2) are critical regulators of the DNA-damage response, controlling cell-cycle checkpoints that may permit recovery from therapy-associated genomic stress. We hypothesized that Chk1/2 inhibition (CHKi) with prexasertib may enhance cytotoxicity from EGFR inhibition plus radiotherapy in head and neck squamous cell carcinoma (HNSCC). In this study, we found that the addition of CHKi to the EGFR inhibitor cetuximab with and without radiotherapy significantly decreased cell proliferation and survival fraction in human papillomavirus virus (HPV)-positive and HPV-negative HNSCC cell lines. Reduced proliferation was accompanied by decreased checkpoint activation, induced S-phase accumulation, persistent DNA damage, and increased caspase cleavage and apoptosis. Importantly, a significant tumor growth delay was observed in vivo in both HPV-positive and HPV-negative cell line xenografts receiving triple combination therapy with CHKi, cetuximab, and radiotherapy without a concomitant increase in toxicity as assessed by mouse body weight. Taken together, the combination of CHKi with cetuximab plus irradiation displayed significant antitumor effects in HNSCCs both in vitro and in vivo, suggesting that this combination therapy may increase clinical benefit. A clinical trial to test this treatment for patients with head and neck cancer is currently ongoing (NCT02555644). Mol Cancer Ther; 16(4); 591-600. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Figures


References
-
- Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–9. - PubMed
-
- Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–78. - PubMed
-
- Chung CH, Ely K, McGavran L, Varella-Garcia M, Parker J, Parker N, et al. Increased epidermal growth factor receptor gene copy numberis associated with poor prognosis in head and neck squamous cell carcinomas. J Clin Oncol. 2006;24:4170–6. - PubMed
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

