Investigating Structure and Dynamics of Proteins in Amorphous Phases Using Neutron Scattering
- PMID: 28138368
- PMCID: PMC5257034
- DOI: 10.1016/j.csbj.2016.12.004
Investigating Structure and Dynamics of Proteins in Amorphous Phases Using Neutron Scattering
Abstract
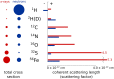
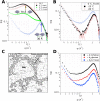
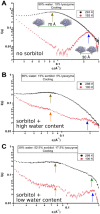
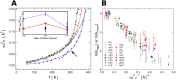
In order to increase shelf life and minimize aggregation during storage, many biotherapeutic drugs are formulated and stored as either frozen solutions or lyophilized powders. However, characterizing amorphous solids can be challenging with the commonly available set of biophysical measurements used for proteins in liquid solutions. Therefore, some questions remain regarding the structure of the active pharmaceutical ingredient during freezing and drying of the drug product and the molecular role of excipients. Neutron scattering is a powerful technique to study structure and dynamics of a variety of systems in both solid and liquid phases. Moreover, neutron scattering experiments can generally be correlated with theory and molecular simulations to analyze experimental data. In this article, we focus on the use of neutron techniques to address problems of biotechnological interest. We describe the use of small-angle neutron scattering to study the solution structure of biological molecules and the packing arrangement in amorphous phases, that is, frozen glasses and freeze-dried protein powders. In addition, we discuss the use of neutron spectroscopy to measure the dynamics of glassy systems at different time and length scales. Overall, we expect that the present article will guide and prompt the use of neutron scattering to provide unique insights on many of the outstanding questions in biotechnology.
Keywords: Freeze-dried proteins; Frozen protein solutions; Glasses; Molecular dynamics; Neutron scattering; Protein dynamics; Protein structure.
Figures






Similar articles
-
Proceedings of the International Workshop on Current Challenges in Liquid and Glass Science, (The Cosener's House, Abingdon 10-12 January 2007).J Phys Condens Matter. 2007 Oct 17;19(41):410301. doi: 10.1088/0953-8984/19/41/410301. J Phys Condens Matter. 2007. PMID: 28192312
-
Small-angle neutron scattering study of protein crowding in liquid and solid phases: lysozyme in aqueous solution, frozen solution, and carbohydrate powders.J Phys Chem B. 2012 Aug 16;116(32):9653-67. doi: 10.1021/jp304772d. Epub 2012 Aug 3. J Phys Chem B. 2012. PMID: 22823457
-
Fundamentals of freeze-drying.Pharm Biotechnol. 2002;14:281-360. doi: 10.1007/978-1-4615-0549-5_6. Pharm Biotechnol. 2002. PMID: 12189727 Review.
-
Drying-induced variations in physico-chemical properties of amorphous pharmaceuticals and their impact on stability (I): stability of a monoclonal antibody.J Pharm Sci. 2007 Aug;96(8):1983-2008. doi: 10.1002/jps.20859. J Pharm Sci. 2007. PMID: 17286290
-
Biomembrane Structure and Material Properties Studied With Neutron Scattering.Front Chem. 2021 Apr 27;9:642851. doi: 10.3389/fchem.2021.642851. eCollection 2021. Front Chem. 2021. PMID: 33987167 Free PMC article. Review.
Cited by
-
Effects of Monovalent Salt on Protein-Protein Interactions of Dilute and Concentrated Monoclonal Antibody Formulations.Antibodies (Basel). 2022 Mar 31;11(2):24. doi: 10.3390/antib11020024. Antibodies (Basel). 2022. PMID: 35466277 Free PMC article.
-
Observation of high-temperature macromolecular confinement in lyophilised protein formulations using terahertz spectroscopy.Int J Pharm X. 2019 Jul 8;1:100022. doi: 10.1016/j.ijpx.2019.100022. eCollection 2019 Dec. Int J Pharm X. 2019. PMID: 31517287 Free PMC article.
-
Tools shaping drug discovery and development.Biophys Rev (Melville). 2022 Jul 27;3(3):031301. doi: 10.1063/5.0087583. eCollection 2022 Sep. Biophys Rev (Melville). 2022. PMID: 38505278 Free PMC article. Review.
-
Hydration-Induced Structural Changes in the Solid State of Protein: A SAXS/WAXS Study on Lysozyme.Mol Pharm. 2020 Sep 8;17(9):3246-3258. doi: 10.1021/acs.molpharmaceut.0c00351. Epub 2020 Aug 19. Mol Pharm. 2020. PMID: 32787275 Free PMC article.
-
Predicting Protein-Protein Interactions of Concentrated Antibody Solutions Using Dilute Solution Data and Coarse-Grained Molecular Models.J Pharm Sci. 2018 May;107(5):1269-1281. doi: 10.1016/j.xphs.2017.12.015. Epub 2017 Dec 21. J Pharm Sci. 2018. PMID: 29274822 Free PMC article.
References
-
- Agorogiannis E.I., Agorogiannis G.I., Papadimitriou A., Hadjigeorgiou G.M. Protein misfolding in neurodegenerative diseases. Neuropathol Appl Neurobiol. 2004;30(3):215–224. - PubMed
-
- Glabe C.G., Kayed R. Common structure and toxic function of amyloid oligomers implies a common mechanism of pathogenesis. Neurology. 2006;66:S74–S78. - PubMed
-
- Vitkup D., Ringe D., Petsko G.A., Karplus M. Solvent mobility and the protein ‘glass' transition. Nat Struct Mol Biol. 2000;7(1):34–38. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
