Decellularized zebrafish cardiac extracellular matrix induces mammalian heart regeneration
- PMID: 28138518
- PMCID: PMC5262469
- DOI: 10.1126/sciadv.1600844
Decellularized zebrafish cardiac extracellular matrix induces mammalian heart regeneration
Abstract
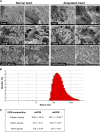
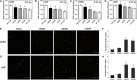
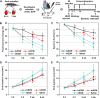
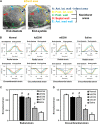
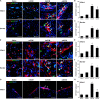
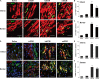
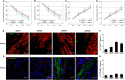
Heart attack is a global health problem that leads to significant morbidity, mortality, and health care burden. Adult human hearts have very limited regenerative capability after injury. However, evolutionarily primitive species generally have higher regenerative capacity than mammals. The extracellular matrix (ECM) may contribute to this difference. Mammalian cardiac ECM may not be optimally inductive for cardiac regeneration because of the fibrotic, instead of regenerative, responses in injured adult mammalian hearts. Given the high regenerative capacity of adult zebrafish hearts, we hypothesize that decellularized zebrafish cardiac ECM (zECM) made from normal or healing hearts can induce mammalian heart regeneration. Using zebrafish and mice as representative species of lower vertebrates and mammals, we show that a single administration of zECM, particularly the healing variety, enables cardiac functional recovery and regeneration of adult mouse heart tissues after acute myocardial infarction. zECM-treated groups exhibit proliferation of the remaining cardiomyocytes and multiple cardiac precursor cell populations and reactivation of ErbB2 expression in cardiomyocytes. Furthermore, zECM exhibits pro-proliferative and chemotactic effects on human cardiac precursor cell populations in vitro. These contribute to the structural preservation and correlate with significantly higher cardiac contractile function, notably less left ventricular dilatation, and substantially more elastic myocardium in zECM-treated hearts than control animals treated with saline or decellularized adult mouse cardiac ECM. Inhibition of ErbB2 activity abrogates beneficial effects of zECM administration, indicating the possible involvement of ErbB2 signaling in zECM-mediated regeneration. This study departs from conventional focuses on mammalian ECM and introduces a new approach for cardiac tissue regeneration.
Keywords: Extracellular matrix; cardiac repair; cardiomyogenesis; decellularization; heart regeneration; ischemic heart disease; myocardial infarction; zebrafish.
Figures

Similar articles
-
Decellularized neonatal cardiac extracellular matrix prevents widespread ventricular remodeling in adult mammals after myocardial infarction.Acta Biomater. 2019 Mar 15;87:140-151. doi: 10.1016/j.actbio.2019.01.062. Epub 2019 Jan 30. Acta Biomater. 2019. PMID: 30710713 Free PMC article.
-
A dual epimorphic and compensatory mode of heart regeneration in zebrafish.Dev Biol. 2015 Mar 1;399(1):27-40. doi: 10.1016/j.ydbio.2014.12.002. Epub 2014 Dec 31. Dev Biol. 2015. PMID: 25557620
-
Human placenta hydrogel reduces scarring in a rat model of cardiac ischemia and enhances cardiomyocyte and stem cell cultures.Acta Biomater. 2017 Apr 1;52:92-104. doi: 10.1016/j.actbio.2016.12.027. Epub 2016 Dec 10. Acta Biomater. 2017. PMID: 27965171
-
How can the adult zebrafish and neonatal mice teach us about stimulating cardiac regeneration in the human heart?Regen Med. 2023 Jan;18(1):85-99. doi: 10.2217/rme-2022-0161. Epub 2022 Nov 23. Regen Med. 2023. PMID: 36416596 Review.
-
Endogenous Mechanisms of Cardiac Regeneration.Int Rev Cell Mol Biol. 2016;326:67-131. doi: 10.1016/bs.ircmb.2016.04.002. Epub 2016 Jul 14. Int Rev Cell Mol Biol. 2016. PMID: 27572127 Review.
Cited by
-
Decellularized extracellular matrix materials for treatment of ischemic cardiomyopathy.Bioact Mater. 2023 Nov 29;33:460-482. doi: 10.1016/j.bioactmat.2023.10.015. eCollection 2024 Mar. Bioact Mater. 2023. PMID: 38076651 Free PMC article. Review.
-
Cardiac regenerative capacity: an evolutionary afterthought?Cell Mol Life Sci. 2021 Jun;78(12):5107-5122. doi: 10.1007/s00018-021-03831-9. Epub 2021 May 5. Cell Mol Life Sci. 2021. PMID: 33950316 Free PMC article. Review.
-
Nitrate-functionalized patch confers cardioprotection and improves heart repair after myocardial infarction via local nitric oxide delivery.Nat Commun. 2021 Jul 23;12(1):4501. doi: 10.1038/s41467-021-24804-3. Nat Commun. 2021. PMID: 34301958 Free PMC article.
-
Processing of Human Cardiac Tissue Toward Extracellular Matrix Self-assembling Hydrogel for In Vitro and In Vivo Applications.J Vis Exp. 2017 Dec 4;(130):56419. doi: 10.3791/56419. J Vis Exp. 2017. PMID: 29286394 Free PMC article.
-
Cellular and Molecular Mechanism of Cardiac Regeneration: A Comparison of Newts, Zebrafish, and Mammals.Biomolecules. 2020 Aug 19;10(9):1204. doi: 10.3390/biom10091204. Biomolecules. 2020. PMID: 32825069 Free PMC article. Review.
References
-
- Li D. Y., Brooke B., Davis E. C., Mecham R. P., Sorensen L. K., Boak B. B., Eichwald E., Keating M. T., Elastin is an essential determinant of arterial morphogenesis. Nature 393, 276–280 (1998). - PubMed
-
- Godwin J., Kuraitis D., Rosenthal N., Extracellular matrix considerations for scar-free repair and regeneration: Insights from regenerative diversity among vertebrates. Int. J. Biochem. Cell Biol. 56, 47–55 (2014). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

