Single-cell analysis reveals a nestin+ tendon stem/progenitor cell population with strong tenogenic potentiality
- PMID: 28138519
- PMCID: PMC5262457
- DOI: 10.1126/sciadv.1600874
Single-cell analysis reveals a nestin+ tendon stem/progenitor cell population with strong tenogenic potentiality
Abstract
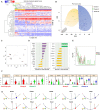
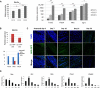
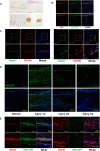
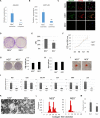
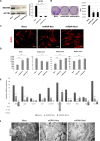
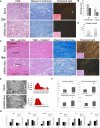
The repair of injured tendons remains a formidable clinical challenge because of our limited understanding of tendon stem cells and the regulation of tenogenesis. With single-cell analysis to characterize the gene expression profiles of individual cells isolated from tendon tissue, a subpopulation of nestin+ tendon stem/progenitor cells (TSPCs) was identified within the tendon cell population. Using Gene Expression Omnibus datasets and immunofluorescence assays, we found that nestin expression was activated at specific stages of tendon development. Moreover, isolated nestin+ TSPCs exhibited superior tenogenic capacity compared to nestin- TSPCs. Knockdown of nestin expression in TSPCs suppressed their clonogenic capacity and reduced their tenogenic potential significantly both in vitro and in vivo. Hence, these findings provide new insights into the identification of subpopulations of TSPCs and illustrate the crucial roles of nestin in TSPC fate decisions and phenotype maintenance, which may assist in future therapeutic strategies to treat tendon disease.
Keywords: Tendons stem/progenitor cells; nestin; single-cell gene analysis; tenogenesis.
Figures

Similar articles
-
Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche.Nat Med. 2007 Oct;13(10):1219-27. doi: 10.1038/nm1630. Epub 2007 Sep 9. Nat Med. 2007. PMID: 17828274
-
Characterization and differentiation of equine tendon-derived progenitor cells.J Biol Regul Homeost Agents. 2011 Apr-Jun;25(2 Suppl):S75-84. J Biol Regul Homeost Agents. 2011. PMID: 22051173
-
AQP1 modulates tendon stem/progenitor cells senescence during tendon aging.Cell Death Dis. 2020 Mar 18;11(3):193. doi: 10.1038/s41419-020-2386-3. Cell Death Dis. 2020. PMID: 32188840 Free PMC article.
-
The Potential Roles of Tendon Stem/Progenitor Cells in Tendon Aging.Curr Stem Cell Res Ther. 2019;14(1):34-42. doi: 10.2174/1574888X13666181017112233. Curr Stem Cell Res Ther. 2019. PMID: 30332976 Review.
-
Targeting Senescent Tendon Stem/Progenitor Cells to Prevent or Treat Age-Related Tendon Disorders.Stem Cell Rev Rep. 2023 Apr;19(3):680-693. doi: 10.1007/s12015-022-10488-9. Epub 2022 Dec 15. Stem Cell Rev Rep. 2023. PMID: 36520409 Review.
Cited by
-
Recombinant fibrous protein biomaterials meet skin tissue engineering.Front Bioeng Biotechnol. 2024 Aug 14;12:1411550. doi: 10.3389/fbioe.2024.1411550. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39205856 Free PMC article. Review.
-
Tendon stem progenitor cells: Understanding the biology to inform therapeutic strategies for tendon repair.J Orthop Res. 2019 Jun;37(6):1270-1280. doi: 10.1002/jor.24156. Epub 2018 Oct 18. J Orthop Res. 2019. PMID: 30270569 Free PMC article. Review.
-
Models of tendon development and injury.BMC Biomed Eng. 2019;1:32. doi: 10.1186/s42490-019-0029-5. Epub 2019 Nov 29. BMC Biomed Eng. 2019. PMID: 32095779 Free PMC article.
-
Vitamin A deficiency causes islet dysfunction by inducing islet stellate cell activation via cellular retinol binding protein 1.Int J Biol Sci. 2020 Jan 30;16(6):947-956. doi: 10.7150/ijbs.37861. eCollection 2020. Int J Biol Sci. 2020. PMID: 32140064 Free PMC article.
-
Biological Therapy for Avascular Necrosis of Femoral Head - A Case Report.J Orthop Case Rep. 2023 Jan;13(1):27-31. doi: 10.13107/jocr.2023.v13.i01.3502. J Orthop Case Rep. 2023. PMID: 37143549 Free PMC article.
References
-
- Gaspar D., Spanoudes K., Holladay C., Pandit A., Zeugolis D., Progress in cell-based therapies for tendon repair. Adv. Drug Deliv. Rev. 84, 240–256 (2015). - PubMed
-
- Bi Y., Ehirchiou D., Kilts T. M., Inkson C. A., Embree M. C., Sonoyama W., Li L., Leet A. I., Seo B.-M., Zhang L., Shi S., Young M. F., Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 13, 1219–1227 (2007). - PubMed
-
- Lovati A. B., Corradetti B., Lange C. A., Recordati C., Bonacina E., Bizzaro D., Cremonesi F., Characterization and differentiation of equine tendon-derived progenitor cells. J. Biol. Regul. Homeost. Agents 25, S75–S84 (2011). - PubMed
-
- Rui Y.-F., Lui P. P. Y., Li G., Fu S. C., Lee Y. W., Chan K. M., Isolation and characterization of multipotent rat tendon-derived stem cells. Tissue Eng. Part A 16, 1549–1558 (2010). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

