GLUT3 upregulation promotes metabolic reprogramming associated with antiangiogenic therapy resistance
- PMID: 28138554
- PMCID: PMC5256137
- DOI: 10.1172/jci.insight.88815
GLUT3 upregulation promotes metabolic reprogramming associated with antiangiogenic therapy resistance
Abstract
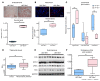
Clinical trials revealed limited response duration of glioblastomas to VEGF-neutralizing antibody bevacizumab. Thriving in the devascularized microenvironment occurring after antiangiogenic therapy requires tumor cell adaptation to decreased glucose, with 50% less glucose identified in bevacizumab-treated xenografts. Compared with bevacizumab-responsive xenograft cells, resistant cells exhibited increased glucose uptake, glycolysis, 13C NMR pyruvate to lactate conversion, and survival in low glucose. Glucose transporter 3 (GLUT3) was upregulated in bevacizumab-resistant versus sensitive xenografts and patient specimens in a HIF-1α-dependent manner. Resistant versus sensitive cell mitochondria in oxidative phosphorylation-selective conditions produced less ATP. Despite unchanged mitochondrial numbers, normoxic resistant cells had lower mitochondrial membrane potential than sensitive cells, confirming poorer mitochondrial health, but avoided the mitochondrial dysfunction of hypoxic sensitive cells. Thin-layer chromatography revealed increased triglycerides in bevacizumab-resistant versus sensitive xenografts, a change driven by mitochondrial stress. A glycogen synthase kinase-3β inhibitor suppressing GLUT3 transcription caused greater cell death in bevacizumab-resistant than -responsive cells. Overexpressing GLUT3 in tumor cells recapitulated bevacizumab-resistant cell features: survival and proliferation in low glucose, increased glycolysis, impaired oxidative phosphorylation, and rapid in vivo proliferation only slowed by bevacizumab to that of untreated bevacizumab-responsive tumors. Targeting GLUT3 or the increased glycolysis reliance in resistant tumors could unlock the potential of antiangiogenic treatments.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

