Sulfmyoglobin Conformational Change: A Role in the Decrease of Oxy-Myoglobin Functionality
- PMID: 28138567
- PMCID: PMC5269605
- DOI: 10.1016/j.bbrep.2016.07.002
Sulfmyoglobin Conformational Change: A Role in the Decrease of Oxy-Myoglobin Functionality
Abstract
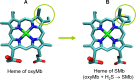
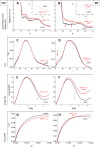
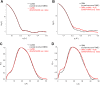
This work is focused at understanding the interaction of H2S with Myoglobin (Mb), in particular the Sulfmyoglobin (SMb) product, whose physiological role is controversial and not well understood. The scattering curves, Guinier, Kratky, Porod and P(r) plots were analyzed for oxy-Mb and oxy-Hemoglobin I (oxyHbI) in the absence and presence of H2S, using Small and Wide Angle X-ray Scattering (SAXS/WAXS) technique. Three dimensional models were also generated from the SAXS/WAXS data. The results show that SMb formation, produced by oxyMb and H2S interaction, induces a change in the protein conformation where its envelope has a very small cleft and the protein is more flexible, less rigid and compact. Based on the direct relationship between Mb's structural conformation and its functionality, we suggest that the conformational change observed upon SMb formation plays a contribution to the protein decrease in O2 affinity and, therefore, on its functionality.
Keywords: SAXS; WAXS; hemoglobin I (HbI); hydrogen sulfide (H2S); myoglobin (Mb); sulfmyoglobin (SMb).
Figures


References
-
- di Masi A., Ascenzi P. H2S: a “double face” molecule in health and disease. BioFactors. 2013;39(2):186–196. - PubMed
-
- Olson K.R., Straub K.D. The role of hydrogen sulfide in evolution and the evolution of hydrogen sulfide in metabolism and signaling. Physiology. 2016;31(1):60–72. - PubMed
-
- Li L., Moore P.K. Putative biological roles of hydrogen sulfide in health and disease: a breath of not so fresh air? Trends Pharmacol. Sci. 2008;29(2):84–90. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
