Prediction of drug-induced liver injury using keratinocytes
- PMID: 28138970
- PMCID: PMC5500258
- DOI: 10.1002/jat.3435
Prediction of drug-induced liver injury using keratinocytes
Abstract
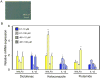
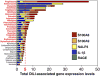
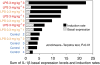
Drug-induced liver injury (DILI) is one of the most common adverse drug reactions. DILI is often accompanied by skin reactions, including rash and pruritus. However, it is still unknown whether DILI-associated genes such as S100 calcium-binding protein A and interleukin (IL)-1β are involved in drug-induced skin toxicity. In the present study, most of the tested hepatotoxic drugs such as pioglitazone and diclofenac induced DILI-associated genes in human and mouse keratinocytes. Keratinocytes of mice at higher risk for DILI exhibited an increased IL-1β basal expression. They also showed a higher inducibility of IL-1β when treated by pioglitazone. Mice at higher risk for DILI showed even higher sums of DILI-associated gene basal expression levels and induction rates in keratinocytes. Our data suggest that DILI-associated genes might be involved in the onset and progression of drug-induced skin toxicity. Furthermore, we might be able to identify individuals at higher risk of developing DILI less invasively by examining gene expression patterns in keratinocytes. Copyright © 2017 John Wiley & Sons, Ltd.
Keywords: IL-1β; drug-induced liver injury; hepatotoxicity; keratinocyte; prediction.
Copyright © 2017 John Wiley & Sons, Ltd.
Conflict of interest statement
The authors did not report any conflict of interest.
Figures


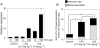
Similar articles
-
Development of a cell-based assay system considering drug metabolism and immune- and inflammatory-related factors for the risk assessment of drug-induced liver injury.Toxicol Lett. 2014 Jul 3;228(1):13-24. doi: 10.1016/j.toxlet.2014.04.005. Epub 2014 Apr 15. Toxicol Lett. 2014. PMID: 24747151
-
Involvement of immune-related factors in diclofenac-induced acute liver injury in mice.Toxicology. 2012 Mar 11;293(1-3):107-114. doi: 10.1016/j.tox.2012.01.008. Epub 2012 Jan 25. Toxicology. 2012. PMID: 22285467
-
Role of chemical structures and the 1331T>C bile salt export pump polymorphism in idiosyncratic drug-induced liver injury.Liver Int. 2013 Oct;33(9):1378-85. doi: 10.1111/liv.12193. Epub 2013 May 23. Liver Int. 2013. PMID: 23701583
-
Case definition and phenotype standardization in drug-induced liver injury.Clin Pharmacol Ther. 2011 Jun;89(6):806-15. doi: 10.1038/clpt.2011.58. Epub 2011 May 4. Clin Pharmacol Ther. 2011. PMID: 21544079 Review.
-
Drug-induced liver injury: what was new in 2008?Expert Opin Drug Metab Toxicol. 2009 Aug;5(8):843-60. doi: 10.1517/17425250903018904. Expert Opin Drug Metab Toxicol. 2009. PMID: 19505188 Review.
Cited by
-
Critical Review of Gaps in the Diagnosis and Management of Drug-Induced Liver Injury Associated with Severe Cutaneous Adverse Reactions.J Clin Med. 2021 Nov 15;10(22):5317. doi: 10.3390/jcm10225317. J Clin Med. 2021. PMID: 34830594 Free PMC article. Review.
-
Composite ammonium glycyrrhizin has hepatoprotective effects in chicken hepatocytes with lipopolysaccharide/enrofloxacin-induced injury.Exp Ther Med. 2020 Nov;20(5):52. doi: 10.3892/etm.2020.9180. Epub 2020 Sep 4. Exp Ther Med. 2020. PMID: 32952642 Free PMC article.
-
HLA-B*57:01-dependent intracellular stress in keratinocytes triggers dermal hypersensitivity reactions to abacavir.PNAS Nexus. 2024 Apr 2;3(4):pgae140. doi: 10.1093/pnasnexus/pgae140. eCollection 2024 Apr. PNAS Nexus. 2024. PMID: 38628599 Free PMC article.
-
Preclinical models of idiosyncratic drug-induced liver injury (iDILI): Moving towards prediction.Acta Pharm Sin B. 2021 Dec;11(12):3685-3726. doi: 10.1016/j.apsb.2021.11.013. Epub 2021 Nov 18. Acta Pharm Sin B. 2021. PMID: 35024301 Free PMC article. Review.
-
Liver-Skin Microphysiological System for Evaluating Topical Drug Delivery-Induced Liver Injury.ACS Omega. 2025 Jun 2;10(23):24284-24295. doi: 10.1021/acsomega.5c00222. eCollection 2025 Jun 17. ACS Omega. 2025. PMID: 40547626 Free PMC article.
References
-
- Barbaud A, Collet E, Milpied B, Assier H, Staumont D, Avenel-Audran M, Grange A, Amarger S, Girardin P, Guinnepain MT, Truchetet F, Lasek A, Waton J. A multicentre study to determine the value and safety of drug patch tests for the three main classes of severe cutaneous adverse drug reactions. Br J Dermatol. 2013;168:555–562. - PubMed
-
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–1425. - PubMed
-
- Bleibel W, Kim S, D’Silva K, Lemmer ER. Drug-induced liver injury. Dig Dis Sci. 2007;52:2463–2471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

