Glaucarubulone glucoside from Castela macrophylla suppresses MCF-7 breast cancer cell growth and attenuates benzo[a]pyrene-mediated CYP1A gene induction
- PMID: 28138972
- PMCID: PMC5435539
- DOI: 10.1002/jat.3436
Glaucarubulone glucoside from Castela macrophylla suppresses MCF-7 breast cancer cell growth and attenuates benzo[a]pyrene-mediated CYP1A gene induction
Abstract
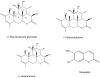
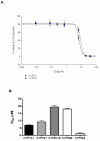
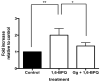
Quassinoids often exhibit antioxidant and antiproliferative activity. Emerging evidence suggests that these natural metabolites also display chemopreventive actions. In this study, we investigated the potential for the quassinoid glaucarubulone glucoside (Gg), isolated from the endemic Jamaican plant Castela macrophylla (Simaroubaceae), to display potent cytotoxicity and inhibit human cytochrome P450s (CYPs), particularly CYP1A enzymes, known to convert polyaromatic hydrocarbons into carcinogenic metabolites. Gg reduced the viability of MCF-7 breast adenocarcinoma cells (IC50 = 121 nm) to a greater extent than standard of care anticancer agents 5-fluorouracil, tamoxifen (IC50 >10 μm) and the tamoxifen metabolite 4-hydroxytamoxifen (IC50 = 2.6 μm), yet was not cytotoxic to non-tumorigenic MCF-10A breast epithelial cells. Additionally, Gg induced MCF-7 breast cancer cell death. Gg blocked increases in reactive oxygen species in MCF-10A cells mediated by the polyaromatic hydrocarbon benzo[a]pyrene (B[a]P) metabolite B[a]P 1,6-quinone, yet downregulated the expression of genes that promote antioxidant activity in MCF-7 cells. This implies that Gg exhibits antioxidant and cytoprotective actions in non-tumorigenic breast epithelial cells and pro-oxidant, cytotoxic actions in breast cancer cells. Furthermore, Gg inhibited the activities of human CYP1A according to non-competitive kinetics and attenuated the ability of B[a]P to induce CYP1A gene expression in MCF-7 cells. These data indicate that Gg selectively suppresses MCF-7 breast cancer cell growth without impacting non-tumorigenic breast epithelial cells and blocks B[a]P-mediated CYP1A induction. Taken together, our data provide a rationale for further investigations of Gg and similar plant isolates as potential agents to treat and prevent breast cancer. Copyright © 2017 John Wiley & Sons, Ltd.
Keywords: breast cancer; chemoprevention; cytochrome P450; cytotoxicity; natural product.
Copyright © 2017 John Wiley & Sons, Ltd.
Figures



Similar articles
-
Rice hull extracts inhibit proliferation of MCF-7 cells with G₁ cell cycle arrest in parallel with their antioxidant activity.J Med Food. 2015 Mar;18(3):314-23. doi: 10.1089/jmf.2014.3181. Epub 2014 Dec 3. J Med Food. 2015. PMID: 25469660
-
Anti-tumor properties of methoxylated analogues of resveratrol in malignant MCF-7 but not in non-tumorigenic MCF-10A mammary epithelial cell lines.Toxicology. 2019 Jun 15;422:35-43. doi: 10.1016/j.tox.2019.04.009. Epub 2019 Apr 18. Toxicology. 2019. PMID: 31004704
-
Carnosic acid inhibits the growth of ER-negative human breast cancer cells and synergizes with curcumin.Fitoterapia. 2012 Oct;83(7):1160-8. doi: 10.1016/j.fitote.2012.07.006. Epub 2012 Jul 22. Fitoterapia. 2012. PMID: 22828666
-
Quassinoids: From traditional drugs to new cancer therapeutics.Curr Med Chem. 2011;18(3):316-28. doi: 10.2174/092986711794839205. Curr Med Chem. 2011. PMID: 21143123 Review.
-
Quassinoid bitter principles. II.Fortschr Chem Org Naturst. 1985;47:221-64. doi: 10.1007/978-3-7091-8790-6_4. Fortschr Chem Org Naturst. 1985. PMID: 3896993 Review. No abstract available.
References
-
- Adams C. Flowering Plants of Jamaica. University Press; Glasgow: 1972. pp. 1–848.
-
- Badal S, Shields M, Niazi U, Jacobs H, Sutcliffe M, Delgoda R. Medimond Publishers. Saratoga Springs; New York, USA: 2008. Screening Natural Products for CYP1 Inhibitors Proceedings of the 17th International Symposium on Microsomes and Drug Oxidation; pp. 20–24.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

