Neutrophil Protease Cleavage of Von Willebrand Factor in Glomeruli - An Anti-thrombotic Mechanism in the Kidney
- PMID: 28139439
- PMCID: PMC5474509
- DOI: 10.1016/j.ebiom.2017.01.032
Neutrophil Protease Cleavage of Von Willebrand Factor in Glomeruli - An Anti-thrombotic Mechanism in the Kidney
Abstract
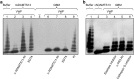
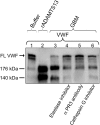
Adequate cleavage of von Willebrand factor (VWF) prevents formation of thrombi. ADAMTS13 is the main VWF-cleaving protease and its deficiency results in development of thrombotic microangiopathy. Besides ADAMTS13 other proteases may also possess VWF-cleaving activity, but their physiological importance in preventing thrombus formation is unknown. This study investigated if, and which, proteases could cleave VWF in the glomerulus. The content of the glomerular basement membrane (GBM) was studied as a reflection of processes occurring in the subendothelial glomerular space. VWF was incubated with human GBMs and VWF cleavage was assessed by multimer structure analysis, immunoblotting and mass spectrometry. VWF was cleaved into the smallest multimers by the GBM, which contained ADAMTS13 as well as neutrophil proteases, elastase, proteinase 3 (PR3), cathepsin-G and matrix-metalloproteinase 9. The most potent components of the GBM capable of VWF cleavage were in the serine protease or metalloprotease category, but not ADAMTS13. Neutralization of neutrophil serine proteases inhibited GBM-mediated VWF-cleaving activity, demonstrating a marked contribution of elastase and/or PR3. VWF-platelet strings formed on the surface of primary glomerular endothelial cells, in a perfusion system, were cleaved by both elastase and the GBM, a process blocked by elastase inhibitor. Ultramorphological studies of the human kidney demonstrated neutrophils releasing elastase into the GBM. Neutrophil proteases may contribute to VWF cleavage within the subendothelium, adjacent to the GBM, and thus regulate thrombus size. This anti-thrombotic mechanism would protect the normal kidney during inflammation and could also explain why most patients with ADAMTS13 deficiency do not develop severe kidney failure.
Keywords: ADAMTS13; Elastase; Glomerular basement membrane; Kidney; Neutrophils; Von Willebrand factor.
Copyright © 2017 The Author(s). Published by Elsevier B.V. All rights reserved.
Figures




References
-
- Benz K., Amann K. Pathological aspects of membranoproliferative glomerulonephritis (MPGN) and haemolytic uraemic syndrome (HUS)/thrombocytic thrombopenic purpura (TTP) Thromb. Haemost. 2009;101:265–270. - PubMed
-
- Berkowitz S.D., Dent J., Roberts J., Fujimura Y., Plow E.F., Titani K., Ruggeri Z.M., Zimmerman T.S. Epitope mapping of the von Willebrand factor subunit distinguishes fragments present in normal and type IIA von Willebrand disease from those generated by plasmin. J. Clin. Invest. 1987;79:524–531. - PMC - PubMed
-
- Camilleri R.S., Cohen H., Mackie I.J., Scully M., Starke R.D., Crawley J.T., Lane D.A., Machin S.J. Prevalence of the ADAMTS-13 missense mutation R1060W in late onset adult thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2008;6:331–338. - PubMed
-
- Dong J.F., Moake J.L., Nolasco L., Bernardo A., Arceneaux W., Shrimpton C.N., Schade A.J., Mcintire L.V., Fujikawa K., Lopez J.A. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002;100:4033–4039. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

