Evidence for ACTN3 as a genetic modifier of Duchenne muscular dystrophy
- PMID: 28139640
- PMCID: PMC5290331
- DOI: 10.1038/ncomms14143
Evidence for ACTN3 as a genetic modifier of Duchenne muscular dystrophy
Abstract
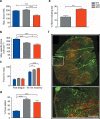
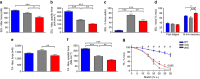
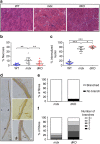
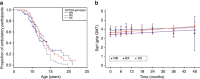
Duchenne muscular dystrophy (DMD) is characterized by muscle degeneration and progressive weakness. There is considerable inter-patient variability in disease onset and progression, which can confound the results of clinical trials. Here we show that a common null polymorphism (R577X) in ACTN3 results in significantly reduced muscle strength and a longer 10 m walk test time in young, ambulant patients with DMD; both of which are primary outcome measures in clinical trials. We have developed a double knockout mouse model, which also shows reduced muscle strength, but is protected from stretch-induced eccentric damage with age. This suggests that α-actinin-3 deficiency reduces muscle performance at baseline, but ameliorates the progression of dystrophic pathology. Mechanistically, we show that α-actinin-3 deficiency triggers an increase in oxidative muscle metabolism through activation of calcineurin, which likely confers the protective effect. Our studies suggest that ACTN3 R577X genotype is a modifier of clinical phenotype in DMD patients.
Conflict of interest statement
E.P.H. has served on advisory committees for AVI BioPharma, Inc., as a consultant with the Gerson Lehman Group, Medacorp, and Lazard Capital, and is cofounder, board member, and shareholder of ReveraGen Biopharma. The remaining authors declare no competing financial interests.
Figures

References
-
- Hoffman E. P., Brown R. H. Jr & Kunkel L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51, 919–928 (1987). - PubMed
-
- Bushby K., Muntoni F., Urtizberea A., Hughes R. & Griggs R. Report on the 124th ENMC International Workshop. Treatment of Duchenne muscular dystrophy; defining the gold standards of management in the use of corticosteroids 2–4 April 2004, Naarden, the Netherlands. Neuromusc. Disord. 14, 526–534 (2004). - PubMed
-
- Moxley R. T. et al. Practice parameter: corticosteroid treatment of Duchenne dystrophy. Neurology 64, 13–20 (2005). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

