Delaying histone deacetylase response to injury accelerates conversion into repair Schwann cells and nerve regeneration
- PMID: 28139683
- PMCID: PMC5290322
- DOI: 10.1038/ncomms14272
Delaying histone deacetylase response to injury accelerates conversion into repair Schwann cells and nerve regeneration
Abstract
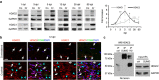
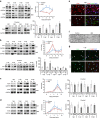
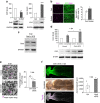
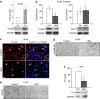
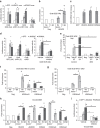
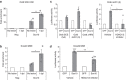
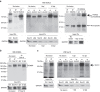
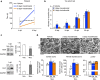
The peripheral nervous system (PNS) regenerates after injury. However, regeneration is often compromised in the case of large lesions, and the speed of axon reconnection to their target is critical for successful functional recovery. After injury, mature Schwann cells (SCs) convert into repair cells that foster axonal regrowth, and redifferentiate to rebuild myelin. These processes require the regulation of several transcription factors, but the driving mechanisms remain partially understood. Here we identify an early response to nerve injury controlled by histone deacetylase 2 (HDAC2), which coordinates the action of other chromatin-remodelling enzymes to induce the upregulation of Oct6, a key transcription factor for SC development. Inactivating this mechanism using mouse genetics allows earlier conversion into repair cells and leads to faster axonal regrowth, but impairs remyelination. Consistently, short-term HDAC1/2 inhibitor treatment early after lesion accelerates functional recovery and enhances regeneration, thereby identifying a new therapeutic strategy to improve PNS regeneration after lesion.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Brosius Lutz A. & Barres B. A. Contrasting the glial response to axon injury in the central and peripheral nervous systems. Dev. Cell 28, 7–17 (2014). - PubMed
-
- Jessen K. R. & Mirsky R. Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia 56, 1552–1565 (2008). - PubMed
-
- Arthur-Farraj P. et al.. The transcription factor c-jun controls Schwann cell demyelination and dedifferentiation after peripheral nerve injury. J. Neuron Glia Biol. 3, S133 (2007).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

