Lysosomal dysfunction and autophagy blockade contribute to IMB-6G-induced apoptosis in pancreatic cancer cells
- PMID: 28139733
- PMCID: PMC5282566
- DOI: 10.1038/srep41862
Lysosomal dysfunction and autophagy blockade contribute to IMB-6G-induced apoptosis in pancreatic cancer cells
Abstract
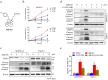
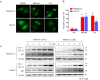
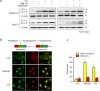
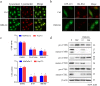
Targeting the autophagic pathway is currently regarded as an attractive strategy for cancer drug discovery. Our previous work showed that IMB-6G is a novel N-substituted sophoridinic acid derivative with potent cytotoxicity against tumor cells, yet the effect of IMB-6G on autophagy and pancreatic cancer cell death remains unknown. Here, we show that IMB-6G inhibits the growth of MiaPaCa-2 and HupT-3 pancreatic cancer cells and induces caspase-mediated apoptosis, which is correlated with an accumulation of autophagic vacuoles. IMB-6G promotes autophagosome accumulation from the early stage of treatment but blocks autophagic flux in the degradation stage, mainly through attenuation of lysosomal cathepsin activity in pancreatic cancer cells. Moreover, IMB-6G triggers lysosomal membrane permeabilization (LMP), followed by cathepsin B/CTSB and cathepsin D/CTSD release from lysosomes into the cytoplasm. Inhibition of autophagosome formation with siRNA against autophagy protein 5 (Atg5) attenuates IMB-6G-induced LMP and apoptosis. Furthermore, cathepsin inhibitors relieve IMB-6G-induced apoptosis as well. Altogether, our findings demonstrate that IMB-6G is a novel autophagy inhibitor, which induces autophagy-dependent apoptosis through autophagosomal-cathepsin axis in pancreatic cancer cells and indicate the potential value of IMB-6G as a novel antitumor drug candidate.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

