Particulate air pollutants, APOE alleles and their contributions to cognitive impairment in older women and to amyloidogenesis in experimental models
- PMID: 28140404
- PMCID: PMC5299391
- DOI: 10.1038/tp.2016.280
Particulate air pollutants, APOE alleles and their contributions to cognitive impairment in older women and to amyloidogenesis in experimental models
Abstract
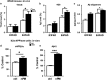
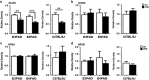
Exposure to particulate matter (PM) in the ambient air and its interactions with APOE alleles may contribute to the acceleration of brain aging and the pathogenesis of Alzheimer's disease (AD). Neurodegenerative effects of particulate air pollutants were examined in a US-wide cohort of older women from the Women's Health Initiative Memory Study (WHIMS) and in experimental mouse models. Residing in places with fine PM exceeding EPA standards increased the risks for global cognitive decline and all-cause dementia respectively by 81 and 92%, with stronger adverse effects in APOE ɛ4/4 carriers. Female EFAD transgenic mice (5xFAD+/-/human APOE ɛ3 or ɛ4+/+) with 225 h exposure to urban nanosized PM (nPM) over 15 weeks showed increased cerebral β-amyloid by thioflavin S for fibrillary amyloid and by immunocytochemistry for Aβ deposits, both exacerbated by APOE ɛ4. Moreover, nPM exposure increased Aβ oligomers, caused selective atrophy of hippocampal CA1 neurites, and decreased the glutamate GluR1 subunit. Wildtype C57BL/6 female mice also showed nPM-induced CA1 atrophy and GluR1 decrease. In vitro nPM exposure of neuroblastoma cells (N2a-APP/swe) increased the pro-amyloidogenic processing of the amyloid precursor protein (APP). We suggest that airborne PM exposure promotes pathological brain aging in older women, with potentially a greater impact in ɛ4 carriers. The underlying mechanisms may involve increased cerebral Aβ production and selective changes in hippocampal CA1 neurons and glutamate receptor subunits.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Selkoe DJ. Preventing Alzheimer's disease. Science 2012; 337: 1488–1492. - PubMed
-
- Farrer LA. Expanding the genomic roadmap of Alzheimer's disease. Lancet Neurol 2015; 14: 783–785. - PubMed
-
- Karagulian F, Belis CA, Dora CFC, Pruss-Ustun AM, Bonjour S, Adair-Rohani H et al. Contributions to cities' ambient particulate matter (PM): A systematic review of local source contributions at global level. Atmos Environ 2015; 120: 475–483.
MeSH terms
Substances
Grants and funding
- HHSN268201100001I/HL/NHLBI NIH HHS/United States
- P50 AG005142/AG/NIA NIH HHS/United States
- HHSN268201100004I/HL/NHLBI NIH HHS/United States
- HHSN268201100046C/HL/NHLBI NIH HHS/United States
- HHSN268201100002C/WH/WHI NIH HHS/United States
- R21 AG050201/AG/NIA NIH HHS/United States
- R21 ES022369/ES/NIEHS NIH HHS/United States
- HHSN268201100001C/WH/WHI NIH HHS/United States
- HHSN268201100004C/WH/WHI NIH HHS/United States
- P30 ES007048/ES/NIEHS NIH HHS/United States
- P30 ES010126/ES/NIEHS NIH HHS/United States
- HHSN268201100003C/WH/WHI NIH HHS/United States
- R01 ES025888/ES/NIEHS NIH HHS/United States
- RF1 AG051521/AG/NIA NIH HHS/United States
- RF1 AG054068/AG/NIA NIH HHS/United States
- R00 AG032361/AG/NIA NIH HHS/United States
- HHSN271201100004C/AG/NIA NIH HHS/United States
- R21 AG040753/AG/NIA NIH HHS/United States
- R01 AG033078/AG/NIA NIH HHS/United States
- HHSN268201100003I/HL/NHLBI NIH HHS/United States
- HHSN268201100002I/HL/NHLBI NIH HHS/United States
- R21 AG040683/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

