Strain-Release Heteroatom Functionalization: Development, Scope, and Stereospecificity
- PMID: 28140573
- PMCID: PMC5334783
- DOI: 10.1021/jacs.6b13229
Strain-Release Heteroatom Functionalization: Development, Scope, and Stereospecificity
Abstract
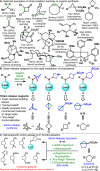
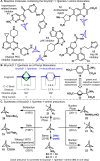
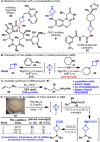
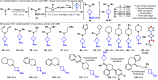
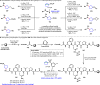
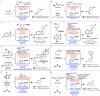
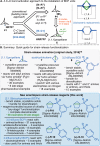
Driven by the ever-increasing pace of drug discovery and the need to push the boundaries of unexplored chemical space, medicinal chemists are routinely turning to unusual strained bioisosteres such as bicyclo[1.1.1]pentane, azetidine, and cyclobutane to modify their lead compounds. Too often, however, the difficulty of installing these fragments surpasses the challenges posed even by the construction of the parent drug scaffold. This full account describes the development and application of a general strategy where spring-loaded, strained C-C and C-N bonds react with amines to allow for the "any-stage" installation of small, strained ring systems. In addition to the functionalization of small building blocks and late-stage intermediates, the methodology has been applied to bioconjugation and peptide labeling. For the first time, the stereospecific strain-release "cyclopentylation" of amines, alcohols, thiols, carboxylic acids, and other heteroatoms is introduced. This report describes the development, synthesis, scope of reaction, bioconjugation, and synthetic comparisons of four new chiral "cyclopentylation" reagents.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


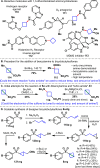


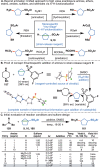
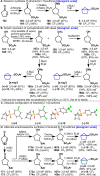
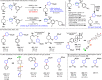
References
-
- Wiberg K. B. Angew. Chem., Int. Ed. Engl. 1986, 25, 312–322. 10.1002/anie.198603121. - DOI
-
- Young I. S.; Kerr M. A. Angew. Chem., Int. Ed. 2003, 42, 3023–3026. 10.1002/anie.200351573. - DOI - PubMed
- Carson C. A.; Kerr M. A. J. Org. Chem. 2005, 70, 8242–8244. 10.1021/jo0512251. - DOI - PubMed
- Dias D. A.; Kerr M. A. Org. Lett. 2009, 11, 3694–3697. 10.1021/ol901454y. - DOI - PubMed
- Humenny W. J.; Kyriacou P.; Sapeta K.; Karadeolian A.; Kerr M. A. Angew. Chem., Int. Ed. 2012, 51, 11088–11091. 10.1002/anie.201206177. - DOI - PubMed
- Grover H. K.; Emmett M. R.; Kerr M. A. Org. Biomol. Chem. 2015, 13, 655–671. 10.1039/C4OB02117G. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

