Intravenous midazolam infusion for sedation of infants in the neonatal intensive care unit
- PMID: 28141899
- PMCID: PMC6464963
- DOI: 10.1002/14651858.CD002052.pub3
Intravenous midazolam infusion for sedation of infants in the neonatal intensive care unit
Abstract
Background: Proper sedation for neonates undergoing uncomfortable procedures may reduce stress and avoid complications. Midazolam is a short-acting benzodiazepine that is used increasingly in neonatal intensive care units (NICUs). However, its effectiveness as a sedative in neonates has not been systematically evaluated.
Objectives: Primary objeciveTo assess the effectiveness of intravenous midazolam infusion for sedation, as evaluated by behavioural and/or physiological measurements of sedation levels, in critically ill neonates in the NICU. Secondary objectivesTo assess effects of intravenous midazolam infusion for sedation on complications including the following.1. Incidence of intraventricular haemorrhage (IVH)/periventricular leukomalacia (PVL).2. Mortality.3. Occurrence of adverse effects associated with the use of midazolam (hypotension, neurological abnormalities).4. Days of ventilation.5. Days of supplemental oxygen.6. Incidence of pneumothorax.7. Length of NICU stay (days).8. Long-term neurodevelopmental outcomes.
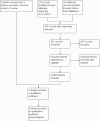
Selection criteria: We selected for review randomised and quasi-randomised controlled trials of intravenous midazolam infusion for sedation in infants aged 28 days or younger.
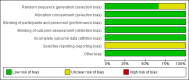
Data collection and analysis: We abstracted data regarding the primary outcome of level of sedation. We assessed secondary outcomes such as intraventricular haemorrhage, periventricular leukomalacia, death, length of NICU stay and adverse effects associated with midazolam. When appropriate, we performed meta-analyses using risk ratios (RRs) and risk differences (RDs), and if the RD was statistically significant, we calculated the number needed to treat for an additional beneficial outcome (NNTB) or an additional harmful outcome (NNTH), along with their 95% confidence intervals (95% CIs) for categorical variables, and weighted mean differences (WMDs) for continuous variables. We assessed heterogeneity by performing the I-squared (I2) test.
Main results: We included in the review three trials enrolling 148 neonates. We identified no new trials for this update. Using different sedation scales, each study showed a statistically significantly higher sedation level in the midazolam group compared with the placebo group. However, none of the sedation scales used have been validated in preterm infants; therefore, we could not ascertain the effectiveness of midazolam in this population. Duration of NICU stay was significantly longer in the midazolam group than in the placebo group (WMD 5.4 days, 95% CI 0.40 to 10.5; I2 = 0%; two studies, 89 infants). One study (43 infants) reported significantly lower Premature Infant Pain Profile (PIPP) scores during midazolam infusion than during dextrose (placebo) infusion (MD -3.80, 95% CI -5.93 to -1.67). Another study (46 infants) observed a higher incidence of adverse neurological events at 28 days' postnatal age (death, grade III or IV IVH or PVL) in the midazolam group compared with the morphine group (RR 7.64, 95% CI 1.02 to 57.21; RD 0.28, 95% CI 0.07 to 0.49; NNTH 4, 95% CI 2 to 14) (tests for heterogeneity not applicable). We considered these trials to be of moderate quality according to GRADE assessment based on the following outcomes: mortality during hospital stay, length of NICU stay, adequacy of analgesia according to PIPP scores and poor neurological outcomes by 28 days' postnatal age.
Authors' conclusions: Data are insufficient to promote the use of intravenous midazolam infusion as a sedative for neonates undergoing intensive care. This review raises concerns about the safety of midazolam in neonates. Further research on the effectiveness and safety of midazolam in neonates is needed.
Conflict of interest statement
E Ng ‐ no conflicts of interest to declare.
A Taddio ‐ no conflicts of interest to declare.
A Ohlsson ‐ no conflicts of interest to declare.
Figures


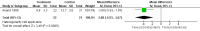












Update of
-
Intravenous midazolam infusion for sedation of infants in the neonatal intensive care unit.Cochrane Database Syst Rev. 2012 Jun 13;(6):CD002052. doi: 10.1002/14651858.CD002052.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2017 Jan 31;1:CD002052. doi: 10.1002/14651858.CD002052.pub3. PMID: 22696328 Updated.
References
References to studies included in this review
Anand 1999 {published data only}
-
- Anand KJS, McIntosh N, Lagercrantz H, Pelausa E, Young TE, Vasa R. Analgesia and sedation in preterm neonates who require ventilatory support ‐ results from the NOPAIN trial. Archives of Pediatrics and Adolescent Medicine 1999;153:331‐8. - PubMed
Arya 2001 {published data only}
-
- Arya V, Ramji S. Midazolam sedation in mechanically ventilated newborns: a double blind randomized placebo controlled trial. Indian Pediatrics 2001;38:967‐72. - PubMed
Jacqz‐Aigrain 1994 {published data only}
-
- Jacqz‐Aigrain E, Daoud P, Burtin P, Desplanques L, Beaufils F. Placebo‐controlled trial of midazolam sedation in mechanically ventilated newborn babies. The Lancet 1994;344:646‐50. - PubMed
References to studies excluded from this review
Kawakami 1998 {published data only}
-
- Kawakami K, Ohata J, Kadosaki M, Saito I, Iwasawa K, Mitono H. Midazolam for anesthetic induction in neonates. Masui‐Japanese Journal of Anesthesiology 1998;47:570‐5. - PubMed
McCarver‐May 1996 {published data only}
-
- McCarver‐May DG, Kang J, Aouthmany M, Elton R, Mowery JL, Slovis TL, et al. Comparison of chloral hydrate and midazolam for sedation of neonates for neuroimaging studies. Journal of Pediatrics 1996;128:573‐6. - PubMed
Parkinson 1997 {published data only}
-
- Parkinson L, Hughes J, Gill A, Billingham I, Ratcliffe J, Choonara I. A randomized controlled trial of sedation in the critically ill. Paediatric Anaesthesia 1997;7:405‐10. - PubMed
Additional references
AAP/CPS 2000
-
- American Academy of Pediatrics, Committee on Fetus and Newborn, Committee on Drugs, Sections on Anesthesiology, Section on Surgery, Canadian Paediatric Society, Fetus and Newborn Committee. Prevention and management of pain and stress in the neonate. Pediatrics 2000;105:454‐61. - PubMed
Adams 1997
-
- Adams MM, Hahn JS, Benitz WE. A series of neonatal patients with paradoxical seizure‐like reactions to bolus intravenous injections of midazolam. Pediatric Research 1997;41:134A.
Ambuel 1992
-
- Ambuel B, Hamlett KW, Marx CM, Blumer JL. Assessing distress in pediatric intensive care environments: the COMFORT scale. Journal of Pediatric Psychology 1992;17:95‐109. - PubMed
Anand 1987
-
- Anand KJ, Hickey PR. Pain and its effects in the human neonate and fetus. New England Journal of Medicine 1987;317:1321‐9. - PubMed
Anand 1992
-
- Anand KJ, Hickey PR. Halothane‐morphine compared with high‐dose sufentanil for anesthesia and postoperative analgesia in neonatal cardiac surgery. New England Journal of Medicine 1992;326:1‐9. - PubMed
Barrier 1989
-
- Barrier G, Attia J, Mayer MN, Amiel‐Tison C, Shnider SM. Measurement of post‐operative pain and narcotic administration in infants using a new clinical scoring system. Intensive Care Medicine 1989;15(Suppl 1):S37‐9. - PubMed
Bergman 1991
-
- Bergman I, Steeves M, Burckart G, Thompson A. Reversible neurologic abnormalities associated with prolonged intravenous midazolam and fentanyl administration. Journal of Pediatrics 1991;119:644‐9. - PubMed
Burtin 1991
-
- Burtin P, Daoud P, Jacqz‐Aigrain E, Mussat P, Moriette G. Hypotension with midazolam and fentanyl in the newborn. The Lancet 1991;337:1545‐6. - PubMed
Carbajal 2015
-
- Carbajal R, Eriksson M, Courtois E, Boyle E, Avila‐Alvarez A, Andersen RD, et al. Sedation and analgesia practices in neonatal intensive care units (EUROPAIN): results from a prospective cohort study. The Lancet Respiriratory Medicine 2015;3(10):796–812. - PubMed
Collins 1991
-
- Collins S, Carter JA. Resedation after bolus administration of midazolam to an infant and its reversal by flumazenil. Anaesthesia 1991;46:471‐2. - PubMed
Craig 1984
-
- Craig KD, McMahon RJ, Morison JD, Zaskow C. Developmental changes in infant pain expression during immunization injections. Social Science and Medicine 1984;19(12):1331‐7. - PubMed
Finnegan 1975
-
- Finnegan LP, Connaughton JF Jr, Kron RE, Emich JP. Neonatal abstinence syndrome: assessment and management. Addictive Diseases 1975;2(1‐2):141‐58. - PubMed
Greenough 1983
-
- Greenough A, Morley C, Davis J. Interaction of spontaneous respiration with artificial ventilation in preterm babies. Journal of Pediatrics 1983;103:769‐73. - PubMed
Harte 1997
-
- Harte GJ, Gray PH, Lee TC, Steer PA, Charles BG. Haemodynamic responses and population pharmacokinetics of midazolam following administration to ventilated, preterm neonates. Journal of Paediatrics and Child Health 1997;33:335‐8. - PubMed
Hartwig 1991
-
- Hartwig S, Roth B, Theisohn M. Clinical experience with continuous intravenous sedation using midazolam and fentanyl in the paediatric intensive care unit. European Journal of Pediatrics 1991;150:784‐8. - PubMed
Hebebrand 1988
-
- Hebebrand J, Hofmann D, Reichelt R, Schnarr S, Knapp M, Propping P, et al. Early ontogeny of the central benzodiazepine receptor in human embryos and fetuses. Life Sciences 1988;43:2127‐36. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Jacqz‐Aigrain 1992
-
- Jacqz‐Aigrain E, Daoud P, Burtin P, Maherzi S, Beaufils F. Pharmacokinetics of midazolam during continuous infusion in critically ill neonates. European Journal of Clinical Pharmacology 1992;42:329‐32. - PubMed
Jacqz‐Aigrain 1996
-
- Jacqz‐Aigrain E, Burtin P. Clinical pharmacokinetics of sedatives in neonates. Clinical Pharmacokinetics 1996;31:423‐43. - PubMed
Koch 2008
-
- Koch SC, Fitzgerald M, Hathway GJ. Midazolam potentiates nociceptive behavior, sensitizes cutaneous reflexes, and is devoid of sedative action in neonatal rats. Anesthesiology 2008;108(1):122–9. - PubMed
Korner 1991
-
- Korner AF, Constantinou J, Dimiceli S, Brown BW, Thom VA. Establishing the reliability and developmental validity of a neurobehavioural assessment for preterm infants: a methodological process. Child Development 1991;62:1200‐8. - PubMed
Korner 1993
-
- Korner AF, Stevenson DK, Kraemer HC, Spiker D, Scott DT, Constantinou J, et al. Prediction of the development of low birth weight preterm infants by a new neonatal medical index. Journal of Developmental and Behavioral Pediatrics 1993;14:106‐11. - PubMed
Lee 1999
-
- Lee TC, Charles BG, Harte GJ, Gray PH, Steer PA, Flenady VJ. Population pharmacokinetic modeling in very premature infants receiving midazolam during mechanical ventilation. Anesthesiology 1999;90:451‐7. - PubMed
Magny 1994
-
- Magny JF, d'Allest AM, Nedelcoux H, Zupan V, Dehan M. Midazolam and myoclonus in neonate. European Journal of Pediatrics 1994;153:389‐92. - PubMed
Marx 1994
-
- Marx CM, Smith PG, Lowrie LH, Hamlett KW, Ambuel B, Yamashita TS, et al. Optimal sedation of mechanically ventilated pediatric critical care patients. Critical Care Medicine 1994;22(1):163‐70. - PubMed
Modanlou 1997
-
- Modanlou HD, Beharry K. Mechanism of midazolam‐induced hypotension: possible role of prostanoids and calcium. Pediatric Research 1997;41:57A.
Ng 2002
-
- Ng E, Klinger G, Shah V, Taddio A. Safety of benzodiazepines in newborns. Annals of Pharmacotherapy 2002;36:1150‐5. - PubMed
Papile 1978
-
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with weights less than 1500 grams. Journal of Pediatrics 1978;92:529‐34. - PubMed
Pellier 1999
-
- Pellier I, Monrigal JP, Moine P, Rod B, Rialland X, Granry JC. Use of intravenous ketamine‐midazolam association for pain procedure in children with cancer. A prospective study. Paediatric Anaesthesia 1999;9:61‐8. - PubMed
Perlman 1985
-
- Perlman JM, Goodman S, Kreusser KL, Volpe JJ. Reduction in intraventricular hemorrhage by elimination of fluctuating cerebral blood‐flow velocity in preterm infants with respiratory distress syndrome. New England Journal of Medicine 1985;312:1353‐7. - PubMed
Quinn 1993
-
- Quinn MW, Wild J, Dean HG, Rushforth JA, Puntis JW, Levene MI. Randomised double‐blind controlled trial of effect of morphine on catecholamine concentrations in ventilated pre‐term babies. The Lancet 1993;342:324‐7. - PubMed
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1.4. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Robieux 1991
-
- Robieaux I, Kumar R, Radhakrishnan S, Koren G. Assessing pain and analgesia with a lidocaine‐prilocaine emulsion in infants and toddlers during venipuncture. Journal of Pediatrics 1991;118(6):971‐3. - PubMed
Rosen 1991
-
- Rosen DA, Rosen KR. Midazolam for sedation in the paediatric intensive care unit. Intensive Care Medicine 1991;17:S15‐9. - PubMed
Schunemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. Grade Working Group. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. www.guidelinedevelopment.org/handbook. Updated 2013.
Snider 2005
-
- Snider L, Tremblay S, Limperopoulos C, Majnemer A, Filion F, Johnston C. Construct validity of the neurobehavioral assessment of preterm infants. Physical & Occupational Therapy in Pediatrics 2005;25(3):81‐95. - PubMed
Stenhammar 1994
Stevens 1996
-
- Stevens BJ, Johnson CC, Petryshen P, Taddio A. Premature Infant Pain Profile: development and initial validation. Clinical Journal of Pain 1996;12:13‐22. - PubMed
The International Neonatal Network
-
- The International Neonatal Network. The CRIB (Clinical Risk Index for Babies) score: a tool for assessing initial neonatal risk and comparing performance of neonatal intensive care units. The Lancet 1993;342:193‐8. - PubMed
van den Anker 1992
-
- Anker JN, Sauer PJJ. The use of midazolam in the preterm neonate. European Journal of Pediatrics 1992;151:152. - PubMed
van Straaten 1992
-
- Straaten HLM, Rademaker CMA, Vries LS. Comparison of the effect of midazolam or vecuronium on blood pressure and cerebral blood flow velocity in the premature newborn. Developmental Pharmacology and Therapeutics 1992;19:191‐5. - PubMed
References to other published versions of this review
Ng 2000
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

