Effects of acute and repeated treatment with the biased mu opioid receptor agonist TRV130 (oliceridine) on measures of antinociception, gastrointestinal function, and abuse liability in rodents
- PMID: 28142305
- PMCID: PMC5646680
- DOI: 10.1177/0269881116689257
Effects of acute and repeated treatment with the biased mu opioid receptor agonist TRV130 (oliceridine) on measures of antinociception, gastrointestinal function, and abuse liability in rodents
Abstract
Rationale: TRV130 (oliceridine; N-[(3-methoxythiophen-2-yl)methyl]-2-[(9 R)-9-pyridin-2-yl-6-oxaspiro[4.5]decan-9-yl]ethanamine) is a novel mu opioid receptor (MOR) agonist that preferentially activates G-protein versus β-arrestin signaling pathways coupled to MORs. Prevailing evidence suggests that TRV130 and other G-protein-biased MOR agonists may produce therapeutic analgesic effects with reduced adverse effects compared to existing MOR agonists.
Objectives: This study compared the effects of acute and repeated TRV130 administration on measures of antinociception, gastrointestinal function, and abuse liability in rodents. We hypothesized that TRV130 would produce robust and sustained antinociception and abuse-related effects during repeated treatment, but that tolerance would develop to gastrointestinal inhibition.
Methods: Antinociception was assessed using a warm-water tail-withdrawal procedure in mice. Gastrointestinal function was assessed in mice using an in vivo measure of fecal output and in vitro assays of colonic propulsion and of colon and ileum circular muscle contraction. Abuse liability was assessed in rats using an intracranial self-stimulation (ICSS) procedure. (+)-TRV130 was administered with acute and repeated dosing regimens, and (-)-TRV130 was also examined in the ICSS procedure to assess stereoselectivity.
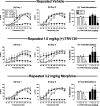
Results: Acute (+)-TRV130 treatment produced robust antinociception, complete inhibition of gastrointestinal function, and weak abuse-related effects. Repeated (+)-TRV130 treatment failed to produce tolerance to antinociception or gastrointestinal inhibition, and abuse-related effects were enhanced by repeated treatment. Effects of acute and repeated (+)-TRV130 in these procedures resemble effects of morphine, with the exception that TRV130 antinociception was more resistant to tolerance. (-)-TRV130 was inactive.
Conclusions: These results suggest that TRV130 retains undesirable constipating and abuse-related effects during repeated treatment despite its bias for G-protein signaling.
Keywords: TRV130; abuse liability; analgesia; constipation; gastrointestinal inhibition; intracranial self-stimulation.
Conflict of interest statement
Figures

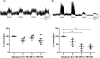


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

