Efficacy of paracetamol, diclofenac and advice for acute low back pain in general practice: design of a randomized controlled trial (PACE Plus)
- PMID: 28143496
- PMCID: PMC5286693
- DOI: 10.1186/s12891-017-1432-5
Efficacy of paracetamol, diclofenac and advice for acute low back pain in general practice: design of a randomized controlled trial (PACE Plus)
Abstract
Background: Low back pain is common and associated with a considerable burden to patients and society. There is uncertainty regarding the relative benefit of paracetamol and diclofenac and regarding the additional effect of pain medication compared with advice only in patients with acute low back pain. This trial will assess the effectiveness of paracetamol, diclofenac and placebo for acute low back pain over a period of 4 weeks. Furthermore, this trial will assess the additional effectiveness of paracetamol, diclofenac and placebo compared with advice only for acute low back pain over a period of 4 weeks.
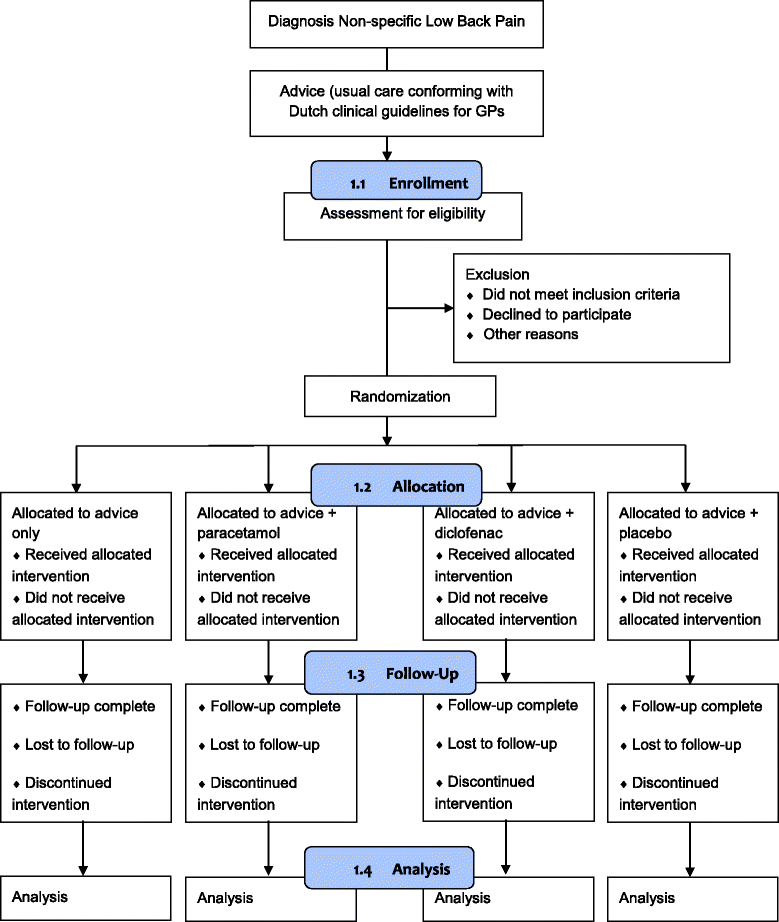
Methods: The PACE Plus trial is a multi-center, placebo-blinded, superiority randomized controlled trial in primary care, with a follow-up of 12 weeks. Patients with acute low back pain aged 18-60 years presenting in general practice will be included. Patients are randomized into four groups: 1) Advice only (usual care conforming with the clinical guideline of the Dutch College of General Practitioners); 2) Advice and paracetamol; 3) Advice and diclofenac; 4) Advice and placebo. The primary outcome is low back pain intensity measured with a numerical rating scale (0-10). Secondary outcomes include compliance to treatment, disability, perceived recovery, costs, adverse reactions, satisfaction, sleep quality, co-interventions and adequacy of blinding. Between group differences for low back pain intensity will be evaluated using a repeated measurements analysis with linear effects models. An economic evaluation will be performed using a cost-effectiveness analysis with low back pain intensity and a cost-utility analysis with quality of life. Explorative analyses will be performed to assess effect modification by predefined variables. Ethical approval has been granted. Trial results will be released to an appropriate peer-viewed journal.
Discussion: This paper presents the design of the PACE Plus trial: a multi-center, placebo-blinded, superiority randomized controlled trial in primary care that will assess the effectiveness of advice only, paracetamol, diclofenac and placebo for acute low back pain.
Trial registration: Dutch Trial Registration NTR6089 , registered September 14th, 2016.
Protocol: Version 4, June 2016.
Keywords: Acetaminophen; Analgesics; Anti-inflammatory agents; Clinical trial; Diclofenac; General practice; General practitioners; Low back pain; Non-steroidal; Pain; Randomized controlled trial; Therapy.
References
-
- Global Burden of Disease Study C Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2015;386(9995):743–800. doi: 10.1016/S0140-6736(15)60692-4. - DOI - PMC - PubMed
-
- Chavannes AW, Mens JMA, Koes BW, Lubbers WJ, Ostelo R, Spinnewijn WEM, Kolnaar BGM. NHG-guideline Non-specific low back pain (in Dutch) Huisarts Wet. 2005;48(3):113–123.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical