ASPirin Intervention for the REDuction of colorectal cancer risk (ASPIRED): a study protocol for a randomized controlled trial
- PMID: 28143522
- PMCID: PMC5286828
- DOI: 10.1186/s13063-016-1744-z
ASPirin Intervention for the REDuction of colorectal cancer risk (ASPIRED): a study protocol for a randomized controlled trial
Abstract
Background: Although aspirin is recommended for the prevention of colorectal cancer, the specific individuals for whom the benefits outweigh the risks are not clearly defined. Moreover, the precise mechanisms by which aspirin reduces the risk of cancer are unclear. We recently launched the ASPirin Intervention for the REDuction of colorectal cancer risk (ASPIRED) trial to address these uncertainties.
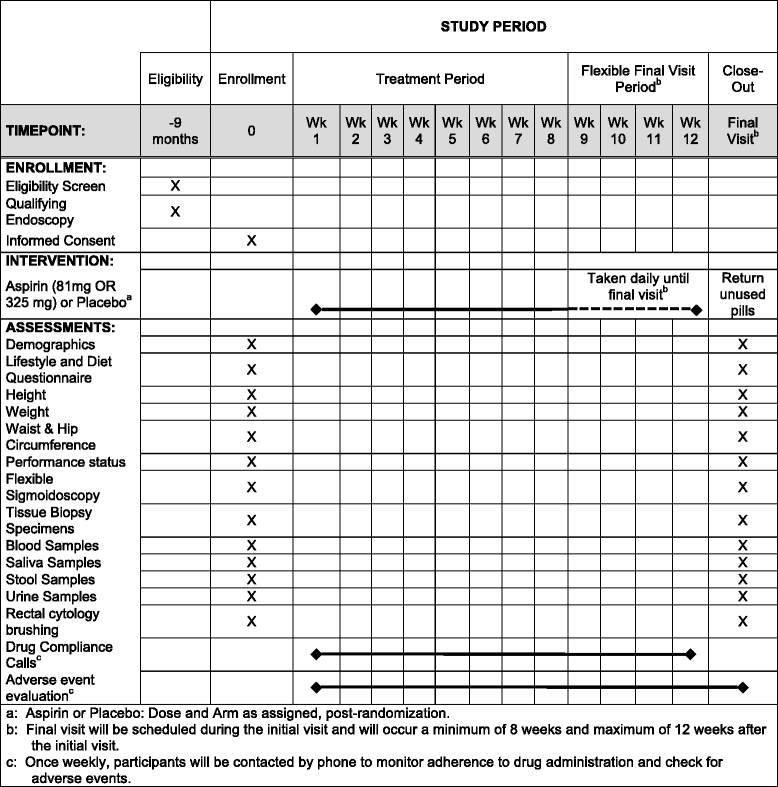
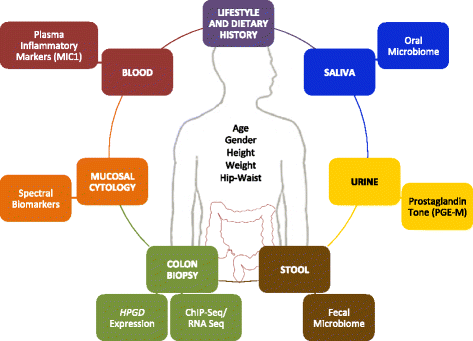
Methods/design: ASPIRED is a prospective, double-blind, multidose, placebo-controlled, biomarker clinical trial of aspirin use in individuals previously diagnosed with colorectal adenoma. Individuals (n = 180) will be randomized in a 1:1:1 ratio to low-dose (81 mg/day) or standard-dose (325 mg/day) aspirin or placebo. At two study visits, participants will provide lifestyle, dietary and biometric data in addition to urine, saliva and blood specimens. Stool, grossly normal colorectal mucosal biopsies and cytology brushings will be collected during a flexible sigmoidoscopy without bowel preparation. The study will examine the effect of aspirin on urinary prostaglandin metabolites (PGE-M; primary endpoint), plasma inflammatory markers (macrophage inhibitory cytokine-1 (MIC-1)), colonic expression of transcription factor binding (transcription factor 7-like 2 (TCF7L2)), colonocyte gene expression, including hydroxyprostaglandin dehydrogenase 15-(NAD) (HPGD) and those that encode Wnt signaling proteins, colonic cellular nanocytology and oral and gut microbial composition and function.
Discussion: Aspirin may prevent colorectal cancer through multiple, interrelated mechanisms. The ASPIRED trial will scrutinize these pathways and investigate putative mechanistically based risk-stratification biomarkers.
Trial registration: This protocol is registered with the U.S. National Institutes of Health trial registry, ClinicalTrials.gov, under the identifier NCT02394769 . Registered on 16 March 2015.
Keywords: Aspirin; Biomarker; Chemoprevention; Colorectal cancer.
Figures
Similar articles
-
Effect of Low-dose and Standard-dose Aspirin on PGE2 Biosynthesis Among Individuals with Colorectal Adenomas: A Randomized Clinical Trial.Cancer Prev Res (Phila). 2020 Oct;13(10):877-888. doi: 10.1158/1940-6207.CAPR-20-0216. Epub 2020 Jul 27. Cancer Prev Res (Phila). 2020. PMID: 32718943 Free PMC article. Clinical Trial.
-
A randomized controlled trial of eicosapentaenoic acid and/or aspirin for colorectal adenoma prevention during colonoscopic surveillance in the NHS Bowel Cancer Screening Programme (The seAFOod Polyp Prevention Trial): study protocol for a randomized controlled trial.Trials. 2013 Jul 29;14:237. doi: 10.1186/1745-6215-14-237. Trials. 2013. PMID: 23895505 Free PMC article. Clinical Trial.
-
Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials.J Natl Cancer Inst. 2009 Feb 18;101(4):256-66. doi: 10.1093/jnci/djn485. Epub 2009 Feb 10. J Natl Cancer Inst. 2009. PMID: 19211452 Free PMC article.
-
Aspirin and other nonsteroidal anti-inflammatory agents in the prevention of colorectal cancer.Important Adv Oncol. 1996:123-37. Important Adv Oncol. 1996. PMID: 8791132 Review.
-
Can We Select Patients for Colorectal Cancer Prevention with Aspirin?Curr Pharm Des. 2015;21(35):5127-34. doi: 10.2174/1381612821666150915111000. Curr Pharm Des. 2015. PMID: 26369678 Review.
Cited by
-
Aspirin enhances the sensitivity of colon cancer cells to cisplatin by abrogating the binding of NF-κB to the COX-2 promoter.Aging (Albany NY). 2020 Jan 6;12(1):611-627. doi: 10.18632/aging.102644. Epub 2020 Jan 6. Aging (Albany NY). 2020. PMID: 31905343 Free PMC article.
-
Aspirin for prevention of colorectal cancer in the elderly: friend or foe?Ann Gastroenterol. 2021;34(1):1-11. doi: 10.20524/aog.2020.0556. Epub 2020 Nov 20. Ann Gastroenterol. 2021. PMID: 33414615 Free PMC article. Review.
-
Mechanisms of Colorectal Cancer Prevention by Aspirin-A Literature Review and Perspective on the Role of COX-Dependent and -Independent Pathways.Int J Mol Sci. 2020 Nov 27;21(23):9018. doi: 10.3390/ijms21239018. Int J Mol Sci. 2020. PMID: 33260951 Free PMC article. Review.
-
Beyond COX-1: the effects of aspirin on platelet biology and potential mechanisms of chemoprevention.Cancer Metastasis Rev. 2017 Jun;36(2):289-303. doi: 10.1007/s10555-017-9675-z. Cancer Metastasis Rev. 2017. PMID: 28762014 Free PMC article. Review.
-
Chemoprevention of Colorectal Cancer.Gastroenterology. 2020 Jan;158(2):368-388. doi: 10.1053/j.gastro.2019.06.047. Epub 2019 Sep 26. Gastroenterology. 2020. PMID: 31563626 Free PMC article. Review.
References
-
- Bibbins-Domingo, K. on behalf of the United States Preventive Services Task Force. Aspirin Use for the Primary Prevention of Cardiovascular Disease and Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164(12):836–45. - PubMed
-
- Neale JR, Dean BJ. Liquid chromatography-tandem mass spectrometric quantification of the dehydration product of tetranor PGE-M, the major urinary metabolite of prostaglandin E(2) in human urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;871:72–7. doi: 10.1016/j.jchromb.2008.06.042. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

